Crystal lattice and unit cell:
Crystalline solid is characterised by
a definite orientation of atoms, ions or
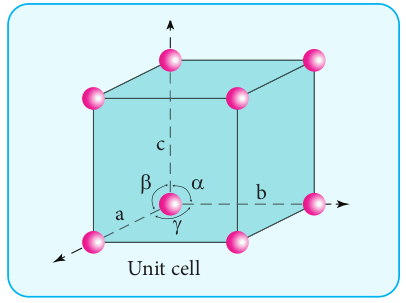
molecules, relative to one another in a three dimensional pattern. The regular arrangement of these species throughout the crystal is called a crystal lattice. A basic repeating structural unit of a crystalline solid is called a unit cell. The following figure illustrates the lattice point and the unit cell.

A crystal may be considered to consist of large number of unit cells, each one in direct contact with its nearer neighbour and all similarly oriented in space. The number of nearest neighbours that surrounding a particle in a crystal is called the coordination number of that particle.
A unit cell is characterised by the three edge lengths or lattice constants a ,b and c and the angle between the edges α, β and γ