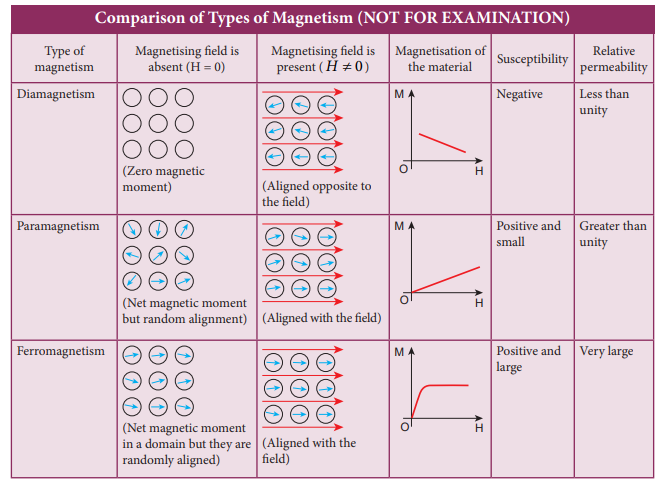
Classification of magnetic materials
The magnetic materials are generally classified into three types based on their behaviour in a magnetising field. They are diamagnetic, paramagnetic and ferromagnetic materials.
(a) Diamagnetic materials
The orbital motion of electrons around the nucleus produces a magnetic field perpendicular to the plane of the orbit. Thus each electron orbit has finite orbital magnetic dipole moment. Since the orbital planes of the other electrons are oriented in random manner, the vector sum of magnetic moments is zero and there is no resultant magnetic moment for each atom.
In the presence of a uniform external magnetic field, some electrons are speeded up and some are slowed down. The electrons whose moments were anti-parallel are speeded up according to Lenz’s law and this produces an induced magnetic moment in a direction opposite to the field. The induced moment disappears as soon as the external field is removed.
When placed in a non-uniform magnetic field, the interaction between induced magnetic moment and the external field creates a force which tends to move the material from stronger part to weaker part of the external field. It means that diamagnetic material is repelled by the field. This action is called diamagnetic action and such materials are known as diamagnetic materials. Examples: Bismuth, Copper and Water etc.
The properties of diamagnetic materials are
-
Magnetic susceptibility is negative.
-
Relative permeability is slightly less than unity.
-
The magnetic field lines are repelled or expelled by diamagnetic materials when placed in a magnetic field.
-
Susceptibility is nearly temperature independent.
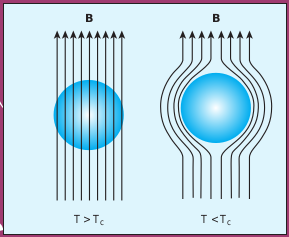
Figure 3.18 Meissner effect – superconductors behave like perfect diamagnetic materials below transition temperature TC.
(b) Paramagnetic materials
In some magnetic materials, each atom or molecule has net magnetic dipole moment which is the vector sum of orbital and spin magnetic moments of electrons. Due to the random orientation of these magnetic moments, the net magnetic moment of the materials is zero.
Magnetic levitated train
Magnetic levitated train is also called Maglev train. This train floats few centimetres above the guideway because of electromagnet used. Maglev train does not need wheels and also achieve greater speed. The basic mechanism of working of Maglev train involves two sets of magnets. One set is used to repel which makes train to float above the track and another set is used to move the floating train ahead at very great speed. These trains are quieter, smoother and environmental friendly compared conventional trains and have potential for moving with much higher speeds with technology in future.

In the presence of an external magnetic field, the torque acting on the atomic dipoles will align them in the field direction. As a result, there is net magnetic dipole moment induced in the direction of the applied field. The induced dipole moment is present as long as the external field exists.When placed in a non-uniform magnetic field, the paramagnetic materials will have a tendency to move from weaker to stronger part of the field. Materials which exhibit weak magnetism in the direction of the applied field are known as paramagnetic materials.
Examples: Aluminium, Platinum, Chromium and Oxygen etc.
The properties of paramagnetic materials are:
- Magnetic susceptibility is positive and small.
- Relative permeability is greater than unity.
- The magnetic field lines are attracted into the paramagnetic materials when placed in a magnetic field.
- Susceptibility is inversely proportional to temperature.
Curie’s law
When temperature is increased, thermal vibration will upset the alignment of magnetic dipole moments. Therefore, the magnetic susceptibility decreases with increase in temperature. In many cases, the susceptibility of the materials is
\[ x_{m} \text{\oe} {\frac{1}{T}} \; or \; x_{m} = {\frac{C}{T}} \]This relation is called Curie’s law. Here C is called Curie constant and temperature T is in kelvin. The graph drawn between magnetic susceptibility and temperature is shown in Figure 3.19, which is a rectangular hyperbola.
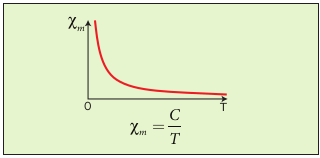
Figure 3.19 Curie’s law – susceptibility vs temperature
(c) Ferromagnetic materials
An atom or a molecule in a ferromagnetic material possesses net magnetic dipole moment as in a paramagnetic material. A ferromagnetic material is made up of smaller regions, called ferromagnetic domains (Figure 3.20). Within each domain, the magnetic moments are spontaneously aligned in a direction. This alignment is caused by strong interaction arising from electron spin which depends on the inter-atomic distance. Each domain has net magnetisation in a direction. However the direction of magnetisation varies from domain to domain and thus net magnetisation of the specimen is zero.
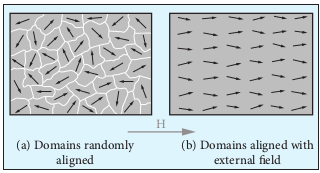
Figure 3.20 Magnetic domains – ferromagnetic materials
In the presence of external magnetic field, two processes take place
-
The domains having magnetic moments parallel to the field grow bigger in size
-
The other domains (not parallel to field) are rotated so that they are aligned with the field.
As a result of these mechanisms, there is a strong net magnetisation of the material in the direction of the applied field (Figure 3.21).
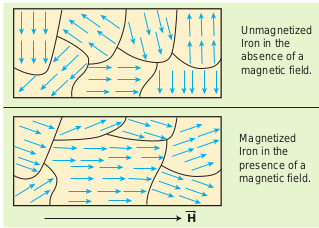
Figure 3.21 Processes of domain magnetization
When placed in a non-uniform magnetic field, the ferromagnetic materials will have a strong tendency to move from weaker to stronger part of the field. Materials which exhibit strong magnetism in the direction of applied field are called ferromagnetic materials. Examples: Iron, Nickel and Cobalt. The properties of ferromagnetic materials are:
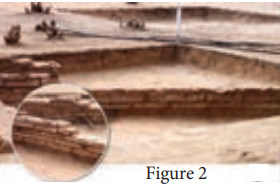
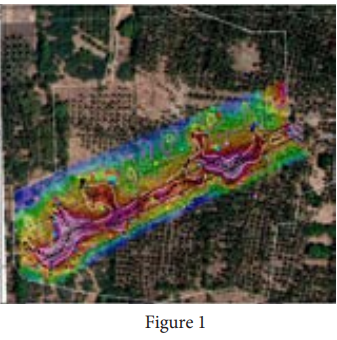
Magnetism plays interesting role in various aspects of life. It has connection with archeological place Keezhadi too. To find whether any archeological structure exists under the surface of a given place, well established technique called ‘magnetometer surveying’ is used. In this technique, the variation of the magnetic field in comparison with the neighbouring place is studied. The magnetic field variation is due to the presence of magnetic mineral magnetite and its related minerals present in the archeological structures like buried wall, pottery, bricks, buried tombs, monuments and inhabited sites. Those minerals are either diamagnetic or paramagnetic or ferromagnetic in nature and each type has different range of magnetic susceptibilities. Indian Institute of Geomagnetism (IIG), Mumbai conducted magnetometer survey on Keezhadi site and found out that there were archeological structures like wall, pottery etc. From the picture (Figure 1), there was magnetic field variation in the range of 10 to 100nT over the particular area (coloured portion). In fact, the existence of massive brick structures at Keezhadi has been revealed through magnetism (Figure 2).
-
Magnetic susceptibility is positive and large.
-
Relative permeability is large.
-
The magnetic field lines are strongly attracted into the ferromagnetic materials when placed in a magnetic field.
-
Susceptibility is inversely proportional to temperature.
Curie-Weiss law
As temperature increases, the ferromagnetism decreases due to the increased thermal agitation of the atomic dipoles. At a particular temperature, ferromagnetic material becomes paramagnetic. This temperature is known as Curie temperature TC. The susceptibility of the material above the Curie temperature is given by
\[x_{m} = {\frac{C}{T-T_{c}}} \]This relation is called Curie-Weiss law. The constant C is called Curie constant and temperature T is in kelvin scale. A plot of magnetic susceptibility with temperature is as shown in Figure 3.22.
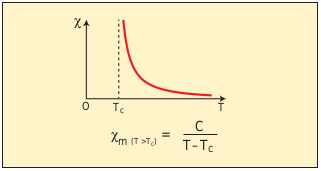
Figure 3.22 Curie-Weiss law – susceptibility vs temperature
Spin
Like mass and charge for particles, spin is also another important attribute for an elementary particle. Spin is a quantum mechanical phenomenon which is responsible for magnetic properties of the material. Spin in quantum mechanics is entirely different from spin we encounter in classical mechanics. Spin in quantum mechanics does not mean rotation; it is intrinsic angular momentum which does not have classical analogue. For historical reason, the name spin is retained. Spin of a particle takes only positive values but the orientation of the spin vector takes plus or minus values in an external magnetic field. For an example, electron has spin s = 1/2. In the presence of magnetic field, the spin will orient either parallel or anti-parallel to the direction of magnetic field.
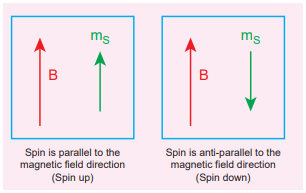
This implies that the magnetic spin ms takes two values for an electron, such as ms = 1 2(spin up) and ms = − 1 2 (spin down). Spin for proton and neutron is s = 1 2. For photon, spin s = 1.