Chapter Outline
7.1 Pathogenic Attributes
7.2 Routes of Entry
7.3 Staphylococcus aureus
7.4 Streptococcus pyogenes
7.5 Neisseria meningitidis
7.6 Corynebacterium diphtheriae
7.7 Clostridium tetani
7.8 Shigella dysenteriae
After studying this chapter the students will be able to,
- Understand the importance of Medical bacteriology.
- Describe the pathogenesis of various bacterial diseases such as skin, respiratory infection, food poisoning, diarrhea and dysentery diseases, STD and zoonotic diseases.
- Know the collection of appropriate clinical specimens and laboratory diagnosis of various bacterial infections.
- Specify how bacterial infections are prevented and treated with appropriate antibiotics.
Learning Objectives
Medica
Chapter
7
7.9 Salmonella typhi
7.10 Vibrio cholerae
7.11 Mycobacterium tuberculosis
7.12 Treponema pallidum
7.13 Leptospira interrogans
l Bacteriology
Medical Bacteriology is the subset of Medical microbiology, which deals with the study of bacterial pathogens. It includes the pathogenesis,
diagnosis, treatment and prevention of various bacterial diseases. Robert Koch is considered as the Father of Bacteriology.
Pathogenic Attributes
The host-parasite relationship is determined by the interaction between host factors and the infecting pathogens. Pathogenicity refers to the ability of a pathogen to produce disease. Virulence is the ability of the pathogen to cause disease.
Adhesion, invasiveness (Streptococcal infections), Bacterial toxins (endotoxins and exotoxins), capsule enzymes (proteases, collagenase, coagulase and other enzymes). These are already explained in the XI Standard text book.
Route of Entry
To establish an infection, pathogen must first enter the host. Normal defense mechanisms and barriers (For example Skin, mucus, ciliated epithelium, lysozyme) make it difficult for the pathogen to enter the body.
Sometimes these barriers are break through for example cut in the skin, wound, tumor, ulcer which provides portal of entry for the bacteria. Some bacterial pathogens have the means to overcome the barriers through various virulence factors and invade the body.
Certain bacteria are infective when introduced through optimal route. The various route of entry of pathogens, which are cut or abrasion or wound (skin), Ingestion, Inhalation, arthropod bite, sexual transmission and congenital transmission. These are already explained in the XI Standard text book. The various bacterial pathogens, its pathogenesis clinical symptoms, laboratory diagnosis, control, prophylaxis and treatment with appropriate antibiotics are discussed below.
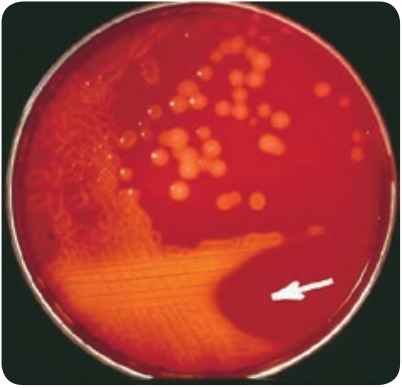
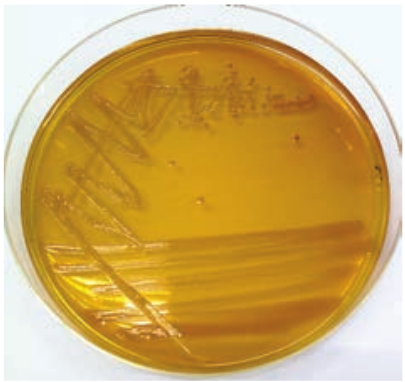
Staphylococcus Aureus (Pyogenic Cocci)
The genus Staphylococcus is included in the family Micrococcaceae. Staphylococcus is a normal flora of skin and mucous membranes, but it accounts for human infections, which is known as staph infection. The name Staphylococcus was derived from a Greek word, ‘staphyle’ means bunch of grapes and ‘kokkos’ means berry. Staphylococcus aureus is a pathogenic species that causes pyogenic infections in human.
Morphology
-
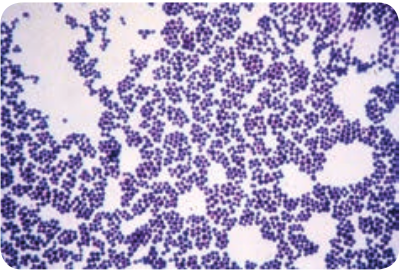
Staphylococci are gram positive spherical cocci, (0.8µm–1.0µm in diameter) arranged characteristically in grape like clusters (Figure 7.1).
-
They are non-motile and non-sporing and few strains are capsulated.
The grape like cluster formation in Staphylococcus aureus is due to cell division
occurring in three perpendicular planes, with daughter cells tending to be remaining in close proximity.
Cultural Characteristics
-
They are aerobes and facultative anaerobes, optimal temperature is 37°C and optimum pH is 7.4–7.6.
-
They grow on the following media and shows the characteristic colony morphology (Table 7.1 & Figure 7.2).
Table 7.1: Staphylococci aureus colony morphology on various media
Media Colony Morphology Nutrient Agar Colonies are circular,
smooth, convex, opaque and produces golden yellow pigment (most strains).
Blood Agar Beta haemolysis
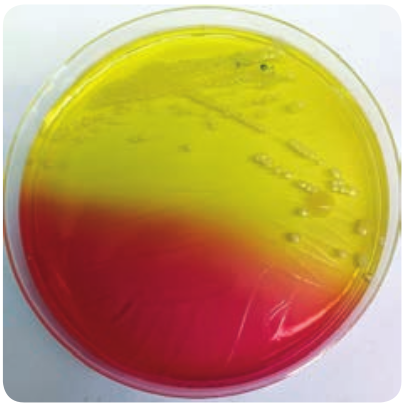
Mannitol salt Agar (MSA)
It is a selective medium for S. aureus produces yellow colored colonies due to fermentation of mannitol
| Me d i a | C ol ony M or ph ol o g y |
|---|---|
| Nut r ien t A ga r | C olo nies a re cir c u l ar,sm o ot h, co nvex, o p aq ueand p ro duces g oldenyel lo w p ig men t (m os tst ra in s). |
| Blo o d A ga r | B et a h aem olysi s |
| Manni tol s a ltAga r (MSA) | It i s a s ele c t ive m e di umfor S. a ure u s p ro ducesyel lo w co lo re d co lo niesdue t o f er men t at io n o fmanni tol |
Virulence Factors
1. Peptidoglycan → It is a polysaccharide polymer. It activates complement and induces the release of inflammatory cytokines.
2. Teichoic acid → it facilitates adhesion of cocci to the host cell surface.
3. Protein A → It is chemotactic, antiphagocytic, anticomplementary and induce platelet injury.
4. Toxins: a. Hemolysins – It is an exotoxin, those
lysis red blood cells. They are of four types namely α-lysin, β-lysin, γ- lysin and delta lysin.
b. Leucocidin – It damages PMNL (polymorphonuclear leucocytes) and macrophages.
c. Enterotoxin – It is responsible for manifestations of Staphylococcus food poisoning.
d. Exfoliative toxin – This toxin causes epidermal splitting resulting in blistering diseases.
e. Toxic shock syndrome toxin – TSST is responsible for toxic shock syndrome.
5. Enzymes: S. aureus produces several enzymes, which are related to virulence of the bacteria. a. Coagulase – It clots human plasma
and converts fibrinogen into fibrin. b. Staphylokinase – It has fibrinolytic
activity. c. Hyaluronidase – It hydrolyzes
hyaluronic acid of connective tissue, thus facilitates the spread of the pathogens to adjacent cells.
d. Other enzymes – S. aureus also produces lipase, nucleases and proteases
Pathogenicity
S. aureus is an opportunistic pathogen which causes infection most commonly at sites of lowered host resistance. (Example: damaged skin)
Mode of Transmission: Staphylococcus infections are transmitted by the following ways.
Why many hospitalized patients are at increased risk for opportunistic infection?
HOTS
Staphylococcus Infections
Direct Contact
Fomites (inanimate objects)
Airborne droplets. (Sneezing or coughing)
Staphylococcal diseases may be classified as
Staphylococcal infection
Cutaneous infections
Deep infections
Food poisoning
Nosocomial infections
Skin exfoliative
diseases
Toxic shock syndrome
(TSS)
It includes the following infections, which are as follows: Cutaneous infections: Wound (injury), burn infections (tissue injury caused by heat), pustules (A small elevated skin lesions containing pus), furuncles (boil forms around a hair follicle and contains
pus), styes (a painful swelling of hair follicle at eyelids), carbuncles (painful cluster of boils of the skin), Impetigo (skin infection with vesicles, pustules which ruptures), pemphigus neonatorum (an auto immune diseases that affect skin and mucous membranes)
Deep infections: It includes Osteomyelitis (inflammation of bones), tonsillitis (inflammation of tonsils), pharyngitis (inflammation of pharynx) sinusitis (inflammation of sinuses), periostitis (inflammation of membrane covering bones), bronchopneumonia (inflammation of lungs), empyema (collection of pus in the body cavity), septicemia (blood poisoning caused by bacteria and its toxins), meningitis (inflammation of meninge), endocarditis (inflammation of endocardium), breast and renal abscess.
Food Poisoning: Staphylococcal food poisoning may follow 2–6 hours after the ingestion of contaminated food (preformed enterotoxin). It leads to nausea, vomiting and diarrhea.
Nosocomial infection: S_. aureus_ is a leading cause of hospital acquired infections. It is the primary cause of lower respiratory tract (LRT) infections and surgical site infections and the second leading cause of nosocomial bacteremia, pneumonia, and Cardiovascular infections.
Exfoliative diseases: These diseases are produced due to the production of epidermolytic toxin. The toxin separates the outer layer of epidermis from the underlying tissues leading to blistering disease. The most dramatic manifestation
| Cut ane ous inf e c t io ns |
|---|
| D e e p inf e c t io ns |
| Foodp oi s on i ng |
| Nos o co mi a linf e c t io ns |
| Sk i n exf oli at ived is e as e s |
| Toxic s ho cks y nd rome(T SS) |
of this toxin is scalded skin syndrome. The patient develops painful rash which slough off and skin surface resembles scalding.
Why most infections acquired through the skin are non-communicable diseases?
HOTS
Toxic shock syndrome toxin: It is caused by TSST-1 and characterized by high fever, hypotension (low blood pressure), vomiting, diarrhea and erythematous rash. TSS became widely known in association with the use of vaginal tampons by menstruating women but it occurs in other situations also.
Laboratory Diagnosis
Specimens: The clinical specimens are collected according to the nature of Staphylococcal infections, which is given in the (Table 7.2).
Table 7.2: Clinical specimen collected for Staphylococcal infections
Infections Clinical Specimens Suppurative lesions
Pus
Respiratory infections
Sputum
Septicemia Blood
Meningitis CSF
Food poisoning Faeces, food or vomitus
Specimens should be transported immediately to the laboratory and processed.
Direct Microscopy: Gram stained smears of clinical specimens is done, where gram positive cocci in clusters were observed. Culture: The collected specimen is inoculated on selective media-MSA and the media incubated at 37°C for 18–24 hours. Next day culture plates are examined for bacterial colonies, which are identified by gram staining, colony morphology and biochemical tests such as a. Catalase test: The genus Staphylococci are
catalase positive. This test distinguishes Staphylococcus from Streptococcus (catalase negative).
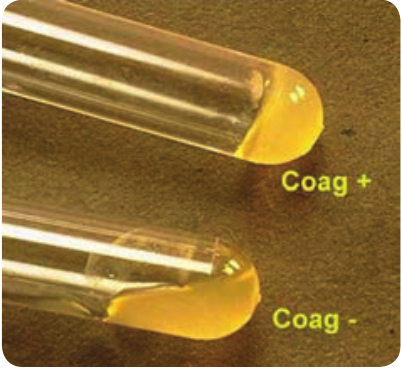
b. Coagulase test: This test helps in differentiating a pathogenic strain from non-pathogenic strain_. S. aureus_ is coagulasepositive (Figure 7.3).
Treatment
Benzyl penicillin is the most effective antibiotic. Cloxacillin is used against beta lactamase. Producing strains (β-lactamase
| Inf e c ti ons | C lini c a l S p e cime ns |
|---|---|
| Suppu r at ivelesio ns | P us |
| R es pira tor yinf e c t io ns | Sputu m |
| S ep t icemi a | Bl ood |
| Menin g it is | C SF |
| Fo o d p ois onin g | Faeces, food or vomitus |
is produced by few strains of S. aureus which cleaves β – lactam ring of penicillin). Vancomycin is used against MRSA (Methicillin Resistant Staphylococcus aureus) strains.
Topical applications: For mild superficial lesions, topical applications of bacitracin or chlorhexidine is recommended.
Control measures: Proper sterilization of medical instruments must be done. Intake of antibiotics must be taken under proper medical advice. The detection of source & carriers among hospital staff, their isolation and treatment should be practiced.
Streptococcus Pyogenes (Flesh eating Bacteria)
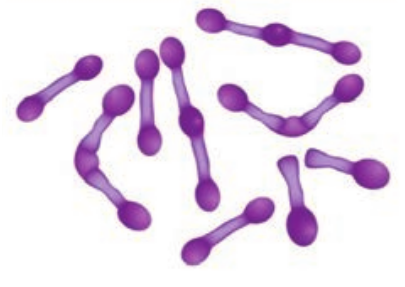
The genus Streptococcus includes a large and varied group of bacteria. They inhabit various sites, notably the upper respiratory tract. However, some species of which Streptococcus pyogenes is the most important and are highly pathogenic. The name Streptococcus is derived from Greek word ‘Streptos’ which means twisted or coiled.
Morphology
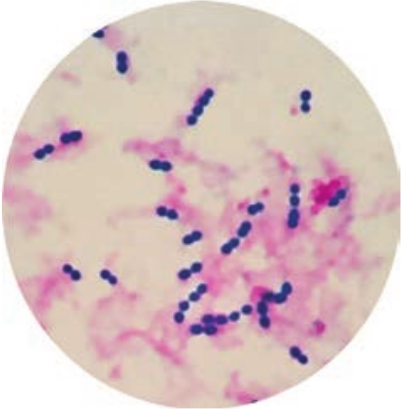
- They are Gram positive, spherical or oval cocci and arranged in chains (0.6µm–1µm)
Year Scientist 1874 The odor Billroth Str 1884 Friedrich Julius
Rosenbach Th an
- They are non – motile, non – sporing. Some strains are capsulated (Figure 7.4).
Cultural Characteristics
-
They are aerobe and facultative anaerobe. Optimum temperature is 37°C and pH is 7.4 to 7.6
-
They grow only in media enriched with blood or serum. It is cultivated on blood agar. On blood agar, the colonies are small, circular, semitransparent, low convex, with an area of clear hemolysis around colonies (Figure 7.5).
-
Crystal violet blood agar – a selective medium for Streptococcus pyogenes.
Contributions eptococci were discovered e cocci were isolated from human lesions d gave the name Streptococcus pyogenes
| Year | S c i en ti st | C ontr i buti on s |
|---|---|---|
| 1874 | Th e o do r B i l lr ot h | Strep toc oc c i w er e di s co ver e d |
| 1884 | Fr ie dr ic h J u li usR os en b ac h | The co cci w ere i s olated f rom human lesio nsand ga ve t he n ame Streptococcu s pyogen es |
Classification
The aerobic and facultative anaerobic Streptoc properties. Three types of haemolytic reactio which are:
Aerobic and Facultative A (Based on Haemoly
Alpha haemolytic
Incomplete haemolytic
Beta haemoly
Comple haemoly
Based on serological grouping of carbo organisms, they are classified into 20 Lancefi
Streptococcus pyogenes is beta haemolytic
Infobi
Antigenic Structure
Capsule: It inhibits phagocytosis Cell wall: The outer layer of cell wall consists of protein and lipoteichoic acid
which helps in attachment to the host cell. Middle layer of cell wall consists of Group Specific C – Carbohydrate that is used for Lancefield grouping. Inner layer of cell wall is made up of peptidoglycan which has pyrogenic and thrombolytic activity. Toxins and Enzymes: Streptococcus pyogenes produces several exotoxins and enzymes which contribute to its virulence. Toxins and Hemolysins: Streptococci produces two types of hemolysins which are Streptolysin O and Streptolysin S. Erythrogenic toxin: (Pyrogenic exotoxin) – The induction of fever is the primary effect of this toxin and it is responsible for the rash of scarlet fever. Enzymes: The various enzyme of _Streptococcus pyogenes_which exhibits virulence activity are listed in Table 7.3.
occus are classified based on haemolytic ns are observed on blood agar medium,
naerobic Streptococci tic properties)
tic
te tic
Gamma haemolytic
No haemolytic
hydrate C antigen of Beta haemolytic eld groups (A to H & K to V) organism which is included in Group A.
ts
| Aer obic a nd F ac u lt at ive A naer obic S t rep to co cci(B as e d o n H aem olyt ic p rop er t ies) | ||||
|---|---|---|---|---|
| ha B elyt ic haem o | Ga mmhaem o | |||
| A lp t a haem o lyt ic | alyt ic |
| A lpha haem olyt ic |
|---|
| Inco mplet ehaem olyt ic |
|——|——| | C omplet ehaem olyt ic |
|——|——| | Nohaem olyt ic |
Table 7.3: Enzymes of Streptococcus pyogenes a
Enzymes streptokinase (fibrinolysin) It promotes t
the convers facilitates the fibrin barrier
Deoxyribonucleases It liquefy the thick pusand streptococca
Hyaluronidase It breaks do favors spread spaces.
Other enzymes NADase, lip other enzym
Streptokinase: It is given intravenously for the treatment of early myocardial
infarction and other thromboembolic disorders. Streptococcus equisimilis is the source of streptokinase used for thrombolytic therapy in patients.
Pathogenesis
Streptococcus pyogenes is intrinsically a much more dangerous pathogen than Staphylococcus aureus and has a much greater tendency to spread in the tissues.
Mode of transmission: Streptococcal infections are transmitted by the following ways:
Transmission of Streptococcal
infection
Direct Contact
Fomites
Airborne droplets
Carriers
nd its virulence nature
Virulence nature he lysis of human fibrin clot by catalyzing ion of plasminogen into plasmin. It spread of infection by breaking down the around the lesions. highly viscous DNA that accumulate in responsible for thin serous character of
l exudates wn hyaluronic acid of the tissues and of streptococcal lesion along intercellular
ase, amylase, esterase, phosphates and es
Streptococcal diseases may be broadly classified, and it is shown in flowchart 7.1
Streptococcal diseases
-
Respiratory tract infection
-
Skin Infection.
-
Streptococcal toxic shock syndrome
-
Deep infection.
-
Genital infections
-
Acute Rheumatic fever
-
Acute glomerulo- nephritis
Suppurative diseases
Non-suppurative complication
Flowchart 7.1: Classification of Streptococcal diseases
Suppurative Infections
1. Respiratory tract infection a. Streptococcal sore throat: Sore throat (acute tonsillitis and pharyngitis) is the
| E nzy mes | Vir u l enc e na tur e |
|---|---|
| st rep tok in as e (f ibr in olysin) | It p romotes t he l ysi s o f hum an f ibr in c lo t by c at a lyzin gt he co nver sio n o f p l asmin og en in to p l asmin. I tfaci li t ates t he spre ad o f inf e c t io n by bre a k in g dow n thef ibr in b ar r ier a roun d t he lesio ns. |
| D e oxy r ib onuc le as es | It liq uef y t he hig h ly v is co us D NA t hat acc um u l ate int hic k p us and r es p onsi ble f or t hin s er ous c harac ter o fst rep to co cc a l exud ates |
| Hya lur onid as e | It b re a ks do w n h ya lur onic acid o f t he t issues a ndfa vors spre ad of st rep to co cc a l lesio n alo ng inter ce l lu l arsp aces. |
| O t her enzy mes | NAD as e, li p as e, a my l as e, es tera s e, p hos phates a ndot her enzy mes |
| Non-s upco mplic | ||||
|---|---|---|---|---|
| Suppu r at ived is e as e s | pura t iveat io n |
| Dir e c t C ont ac t |
|---|
| Fomites |
| Airb or ne dr oplets |
| C ar r ier s |
most common streptococcal diseases. Tonsillitis is more common in older children and adults. The pathogen may spread from throat to the surrounding tissues leading to suppurative (pus – formation) complication such as cervical adenitis (inflammation of a lymph node in the neck) otitis media (inflammation of middle ear), quinsy (ulcers of tonsils) Ludwig’s angina (purulent inflammation around the sub maxillary glands) mastoiditis (inflammation of mastoid process). b. Scarlet fever: The disease consists of combination of sore throat and a generalized erythematous (redness of skin or mucous membranes) rash.
2. Skin infections a. Erysipeals: It is an acute spreading lesion. The skin shows massive brawny oedema with erythema it is seen in elderly persons or elders. b. Impetigo: (Streptococcal pyoderma) It is a skin infection that occurs most often in young children. It consists of superficial blisters that break down and eroded areas whose surface is covered with pus. It is the main cause leading to acute glomerulonephritis in children. c. Necrotizing fasciitis: It is an invasive, infection characterized by inflammation and necrosis of the skin, subcutaneous fat and fascia. It is a life-threatening infection.
The strain which cause necrotizing fasciitis to have been named as “Flesh
eating bacteria or” killer bacteria.
3. Streptococcal toxic shock syndrome Streptococcal pyrogenic exotoxin leads to streptococcal toxic shock syndrome (TSS). It is a condition in which the entire organ system collapses, leading to death.
4. Genital infections Streptococcus pyogenes is an important cause of puerperal sepsis or child bed fever (infection occur when bacteria infect the uterus following child birth)
5. Deep infection Streptococcus pyogenes may cause pyaemia (blood poisoning characterized by pus forming pathogens in the blood) septicemia (A condition in which bacteria circulate and actively multiply in the bloodstream) abscess in internal organs such as brain, lung, liver and kidney.
Non – Suppurative Complication
Streptococcus pyogenes infections are sometimes followed by two important non – suppurative complications which are, acute rheumatic fever and acute glomerulonephritis. These complications occur 1–4 weeks after the acute infection and it is believed to be the result of hypersensitivity to some streptococcal components.
1. Rheumatic fever It is often preceded by sore throat and most serious complication of haemolytic streptococcal infection. The mechanism by which Streptococci produce rheumatic fever is still not clear. A common cross – reacting antigen exist in some group A streptococci and heart, therefore, antibodies produced in response to the
streptococcal infection could cross react with myocardial and heart valve tissue, causing cellular destruction.
2. Acute glomerulonephritis It is often preceded by the skin infection. It is caused by only a few “nephritogenic types (strains)”. It develops because some components of glomerular basement membrane are antigenically similar to the cell membranes of nephritogenic streptococci. The antibodies Formed against Streptococci cross react with glomerular basement membrane and damage. Some patients develop chronic glomerulonephritis with ultimate kidney failure.
Why are some staphylococcal skin infections similar to streptococcal skin infection?
HOTS
Laboratory Diagnosis
Specimens: Clinical specimens are collected according to the site of lesion. Throat swab, pus or blood is obtained for culture and serum for serology. Direct Microscopy: Gram stained smears of clinical specimens is done, where Gram positive cocci in chains were observed. It is indicative of streptococcal infection. Culture: The clinical specimen is inoculated on blood agar medium and incubated at 37° C for 18–24 hours. After incubation period, blood agar medium was observed for zone of beta – haemolysis around colonies. Catalase test: Streptococci are catalase negative which is an important
test to differentiate Streptococci from_Staphylococci_. Serology: Serological tests are done for rheumatic fever and glomerulonephritis. It is established by demonstrating high levels of antibody to streptococci toxins. The standard test is antistreptolysin Otitration. ASOtitres higher than 200 units are indicative of prior Streptococcal infection.
Treatment and Prophylaxis
- Penicillin G is the drug of choice.
- In patients allergic to penicillin,
erythromycin or cephalexin is used.
- Antibiotics have no effect on
established glomerulonephritis and rheumatic fever.
-
Prophylaxis is indicated only in the prevention of rheumatic fever, it prevents streptococcal reinjection and further damage to the heart.
-
Penicillin is given for a long period in children who have developed early signs of rheumatic fever.
Myth: Eating choc- olate encourages the development of acne.
Fact: It is the oils and fats in many chocolate products, and not chocolate itself, that promote sebum production and subsequent acne. Chocolate in low-fat chocolate milk and in fat-free chocolate candies does not encourage acne. Acne suffers do not need to give up chocolate, they need to reduce their lipid consumption.
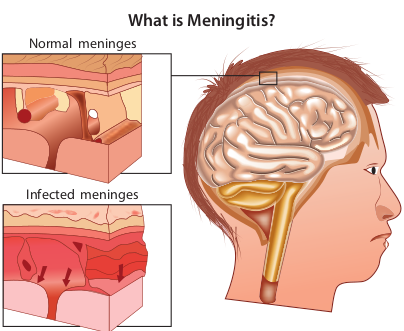
Neisseria Meningitides (Meningococcus)
The genus Neisseria is included in the family Neisseriaceae (Figure 7.6). It contains two important pathogens Neisseria meningitidis and Neisseria gonorrhoeae, both the species are strict human pathogens. N. meningitides causes meningococcal meningitis (formerly known as cerebrospinal fever).
The word Meningitis is derived from Greek word ‘meninx’ means membrane and ‘itis’ means inflammation. It is an inflammation of meanings of brain or spinal cord. Bacterial meningitis is a much more severe disease than viral meningitis.
Morphology
They are Gram negative diplococci (0.6µm–0.8µm in size) arranged typically in pairs, with adjacent sides flattened.
They are non – motile, capsulated (Fresh isolates).
Cocci are generally intracellular when isolated from lesions (Figure 7.7).
Cultural Characteristic
They are strict aerobes, but growth is facilitated by 5–10% CO2 and high
Table 7.4: Colony morphology of Neisseria Meningitides on media
Name of the media
Colony Morphology
Chocolate agar Colonies are large, colorless to grey opaque colonies.
Mueller Hinton agar
Colonies are small, round, convex grey, translucent with entire edges. The colonies are butyrous in consistency and easily emulsified.
humidity. The optimum temperature is 35°C–36°C and optimum pH is 7.4–7.6. They are fastidious pathogens, growth occurs on media enriched with blood or serum. They grow on the following media and show the characteristic colony morphology (Table 7.4).
Pathogenesis
N. meningitidis is the causative agent of meningococcal meningitis, also known as pyogenic or septic meningitis. Infection is most common in children
Normal meninges
Infected meninges
What is Meningitis?
| Name o f theme di a | C o l ony Mor ph ol o g y |
|---|---|
| C ho co l ate a ga r | C olo nies a re l arge,co lo rles s t o g re yop aq ue co lo nies. |
| Mue l ler H in tonag ar | C olo nies a re sm a l l,roun d, co nvex g re y,t ra nslucen t w it hen t ir e e dg es. Th eco lo nies are but y rousin co nsi sten c y a nde asi ly em u lsif ie d. |
and young adults. Meningococci are strict human pathogens. Human nasopharynx is the reservoir of N.meningitidis. The pathogenesis is dicussed in the flowchart 7.2. Source of infection – Airborne droplets Route of entry – Nasopharynx Site of infection – Meninges Incubation period – 3 days
Laboratory Diagnosis
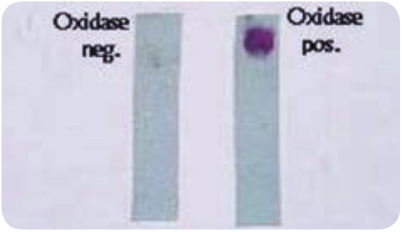
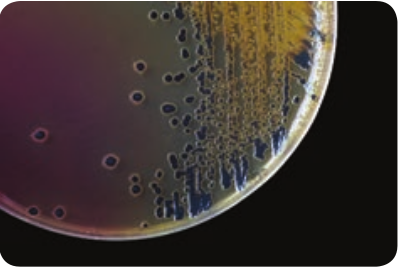
Specimens: CSF, blood, nasopharyngeal scrapings from petechiae lesions are the specimens collected from pyogenic meningitis patients. Direct Microscopy: CSF is centrifuged, and smear is prepared from the deposit for gram staining. Meningococci are Gram negative diplococci, present mainly inside polymorphs and many pus cells are also seen. Culture: The centrifuged deposit of CSF is inoculated on chocolate agar. The plate is incubated at 36°C under 5–10% CO2 for 18–24 hours. After incubation period, meningococcusis identified by gram staining, colony morphology and biochemical reactions. N. meningitides is catalase and oxidase positive (Figure 7.8).
The meningococci gain entry in the nasopharynx and attaches to the epithelial cells with pili. They are engulfed by epithelial cells of mucosa and penetratesintonearby blood vessels, thereby damaging the epithelium and causes pharyngitis.
Cocci spread from the nasopharynx to meninge by travelling along the perineural sheath of the olfactory nerve, through the cribriform plate to the subarachnoid space or through blood stream.
Pathogen entering the blood vessels rapidly permeates the meninges and produce meningitis (most complication in children). It is marked by the following clinical manifestations, which are fever, sore throat, headache, stiff neck, and vomiting convulsions (fits).
The pathogen sheds endotoxin into the generalized circulation, which damages the blood vessels and leads to vascular collapse, hemorrhage, petechiae lesion (a small red or purple spot caused by bleeding into the skin).
A few develop sudden meningococcemia (water house – friderichsen syndrome) characterized by shock, disseminated intravascular coagulation and multisystem failure.
It has a violet onset, with fever, chills, shock and coma. Generalized intravascular clotting, cardiac failure, damage to adrenal glands and death occurs within a few hours.
Flowchart 7.2: Pathogenesis of Neisseria Meningitides
Treatment and Prophylaxis
Penicillin – G is the drug of choice. In penicillin allergic cases, chloramphenicol is recommended.
- Monovalent and polyvalent vaccines
(capsular polysaccharide) induce good immunity in older children and adults.
- Conjugate vaccines are used for children below the age of 2 years.
Corynebacterium Diphtheriae
Several species of the genus Corynebacterium are normal flora of skin, upper respiratory tract (URT), urogenital and intestinal tract. The most important member of the genus is C. diphtheriae the causative agent of diphtheria, a localized inflammation of the throat with greyish white pseudomembrane and a generalized toxemia due to the secretion and dissemination of a highly potent toxin.
The name Corynebacterium diphtheria is derived from Greek word ‘Coryne’ – “Club shaped swellings” or“Knotted rod” ‘Diphthera’ – Leather.
Morphology
- They are Gram positive slender rods, pleomorphic club shape or coryneform
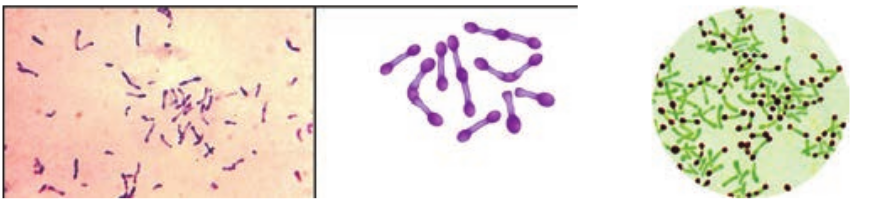
bacterium Non – motile, non – sporing and non – capsulated (Figure 7.9a & b).
-
The bacilli are arranged in a characteristic fashion in angular fashion resembling the letters V or L. This has been called Chinese letter or cuneiform arrangement (Figure 7.10).
-
They are club shaped due to the presence of metachromatic granules at one or both ends. These granules are composed of polymetaphosphates and represent energy storage depots.
ium diphtheriae (b) Albert’s staining
Cultural Characteristics
-
They are aerobic and facultative anaerobe. Optimum temperature is 37°C and pH 7.2.
-
They grow on the following media and show the characteristic colony morphology (Table 7.5).
Table 7.5: Colony Morphology of Corynebacterium diphtheriae on cultural media
Media Colony Morphology Loeffler’s Serum slope
They grow on this medium very rapidly. Colonies appear after 6–8 hours of incubation. The colonies are small, circular white or creamy and glistening
Tellurite Blood Agar
Grey or black colonies. Based on colony morphology on tellurite medium, three main biotypes – Gravis, Intermedius and Mitis.
*Toxin.
- The pathogenicity is due to production
of a very powerful exotoxin by virulent strains of diphtheria bacilli.
- The toxigenicity of diphtheria bacillus depends on the presence of a tox+ gene which can be transferred from one bacterium to another by lysogenic bacteriophages, of which beta phage is the most important.
Properties The diphtheria toxin is a heat – labile protein and has a molecular weight of about 62,000 Dalton. It consists of two fragments.
a. Fragment A (24,000 Dalton) – It has all enzymatic activity.
b. Fragment B (38,000 Dalton) – It is responsible for binding the toxin to the target cells.
Mode of Action The toxin acts by inhibiting protein synthesis, specifically fragment A inhibits polypeptide chain elongation in the presence of NAD by inactivating the elongation factor (EF – 2) the toxin has special affinity for myocardium, adrenal gland and nerve endings.
Pathogenicity
Source of infection – Airborne droplets Route of entry – Upper respiratory tract Incubation period – 3–4 days Site of infection – Faucial (nasal, otitis, conjunctival, laryngeal, genital) diphtheria is most commonly seen in children of 2–10years.
Faucial diphtheria is the most common type. The infection is confined to humans only. The toxin has both local (flowchart 7.3) as well as systemic effects.
Systemic effects The toxin diffuses into the blood stream and causes toxemia. It has got affinity for cardiac muscle, adrenal and nerve endings. It acts on the cells of these tissues.
Clinical Manifestations
1. Laryngeal obstruction, asphyxia (it is a condition of severe deficient supply of oxygen, causing suffocation).
2. Diphtheritic myocarditis (inflammation of heart muscle), polyneuropathy
| Me d i a | C ol ony M or ph ol o g y |
|---|---|
| L o ef f ler ’sS er um s lo p e | Th e y g row o n t hi sme di um v er y ra pid ly.C olo nies a pp e ar a f ter6–8 h our s o f in c ub at io n.Th e co lo nies a re sm a l l,cir c u l ar w hi te o r cr e amyand g li stenin g |
| Tel lu r it eBlo o d A ga r | Gre y o r b l ac k co lo nies.B as e d o n co lo nymor pholog y o n t el lur iteme di um, t hr e e m ainbio t yp es – G ra v is,Inter me di us a nd M it is. |
(damage of multiple peripheral nerves), paralysis of palatine (the top part of the inside of the mouth) and ciliary muscles.
3. Degenerative changes in adrenal glands, kidney and liver may occur.
Laboratory Diagnosis
Specimen: Two swabs from the lesions are collected. One swab is used for smear preparationand other swab for inoculation on culturemedia. Direct microscopy: Smears are stained with both Gram stain and Albert stain. a. Gram Staining – Gram positive slender
rods were observed. b. Albert staining – Club shaped with
metachromatic granules were observed.
The bacilli enters through URT and it remains confined to the site of entry where they multiply and produces diphtheria toxin
The membrane extends from oropharynx to larynx and into trachae. The mechanical obstruction of the trachae by psedudomembrane can cause suffocation and death.
The toxin causes local necrotic changes along with superficial inflammatory reaction. The necrosed epithelium together with fibrinous exudate, leucocytes, erthrocytes and bacteria consitute the greyish psedudomembrane, which is a characteristic feature of diphtheritic infection.
Flowchart 7.3: Localized effect of diphtheria toxin
Culture: The swabis inoculated on Loeffler’s serum slope, after overnight incubation at 37°C, the plates were observed for characteristic colonies, which are identified by gram staining.
Prophylaxis
Diphtheria can be controlled by immunization. Three methods of immunization are available (Table 7.6).
Treatment
The specific treatment for diphtheria consists of administration of antitoxin with dose of 20,000–100, 000 units of ADS intramuscularly and antibiotic therapy using penicillin.
Clostridium Tetani
The genus Clostridium consists of anaerobic, spore forming Gram positive bacilli. The spores are wider than the bacterial bodies, giving the bacillus a swollen appearance resembling a spindle. The name Clostridium is derived from the word ‘kluster’ (a spindle). Most species are saprophytes found in soil, water and decomposing plant and animal matter. Some of the pathogens are normal flora of intestinal tract of human and animals.
The genus Clostridium includes bacteria that causes 3 major diseases of human – Tetanus, gas gangrene and food poisoning. Clostridium pathogenicity is mainly due to production of a powerful exotoxin.
Clostridium of medical importance may be classified based on diseases they produce, which is given the Table (7.7).
Table 7.7: Clostridium sp. causing pathogenic diseases.
Organisms Diseases Clostridium tetani Tetanus Clostridium perfringens Gas gangrene Clostridium botulinum Food poisoning
Morphology
They are Gram positive spore forming rods. The spores are spherical and terminal in position giving a drumstick appearance. They are motile and non – capsulated.
Culture Characteristics
-
They are obligate anaerobes, optimum temperature is 37°C and pH is 7.4.
-
It grows on ordinary media, but growth is enhanced by addition of blood and serum. Clostridia tetani grows on the following media and show the characteristic colony morphology (Table 7.8).
Toxins
Clostridium tetani produces two distinct toxins namely, a. Tetanolysis (haemolysin) b. Tetanospasmin (neurotoxin)
Table 7.6: Immunization for diphtheria
Immuniz Active Passive DPT (Triplevaccine) 3 doses of 0.5ml each are given intramuscular route at an interval of 4–6 weeks after birth. Booster dose of DPT are given at 18 months and at the age of 5.
500–1000 units theritic serum, ( ministered subc
*Tetanolysis.
- Heat labile and oxygen labile toxin.
- It lysis erythrocytes and also acts as
neurotoxin.
*Tetanospasmin.
- It is heat labile and oxygen stable
powerful neurotoxin.
- It is protein in nature. consisting of
a large polypeptide chain (93,000 Dalton) and a smaller polypeptide chain (52,000 Dalton) joined by a disulphide bond.
- Mode of Action: Tetanospasminis a neurotoxin, which blocks the release of
ation Combined
of Antidiph- ADS) is ad- utaneously.
It consists of administration first dose of adsorbed toxoid on one arm, while ADS is given on another arm.
Table 7.8: Colony characteristics of Clostridium tetani
Media Colony Morphology Blood agar They produce α – hemolysis
which subsequently develop into β – hemolysis (due to tetanolysis) it produces swarming growth.
Cooked meat broth (CMB)
Growth occurs as turbidity with gas formation. The meat is not digested but becomes black on prolonged incubation
| Imm uniza ti on | ||
|---|---|---|
| Ac ti ve | Pass iv e | C om bi n e d |
| DPT (Tr iple vaccin e)3 dos es o f 0.5m l e ac h a reg iven in t ra mus c u l ar r outeat a n in ter va l o f 4–6 w e eksaf ter b ir t h. B o os ter dos e o fDPT are g iven at 18 mont hsand a t t he a ge o f 5. | 500–1000 uni ts of Ant idi ph -t her it ic s er um, (ADS) i s ad -mini ster e d s ub c ut ane ously. | It co nsi sts o f admini st ra t io nf ir st dos e of ads orb e d toxoidon o ne a r m, w hi le ADS i sg iven o n a not her a r m. |
| Org anis ms | Dis e as es |
|---|---|
| C lostr idium t etani | Tet anus |
| Clostridium per fringens | Ga s ga ng ren e |
| C lostr idium b otulinum | Fo o d p ois onin g |
| Me d i a | C ol ony M or ph ol o g y |
|---|---|
| Blo o d a ga r | Th e y p ro duce α – h em olysi sw hic h s ubs e quen t ly de velo pinto β – h em olysi s (d ueto t et anolysi s) i t pro ducesswa r min g g rowt h. |
| C o oke d mea t b roth(CMB) | Growt h o cc ur s a s turb idi t yw it h ga s for mat io n. Th eme at i s n ot dig es te d b utb e co mes bl ac k on prolo nge di nc ub at i on |
inhibitory neurotransmitters (glycine and gamma – amino butyric acid) across the synaptic junction. The toxin acts presynaptically, the abolition of spinal inhibition causes uncontrolled spread of impulses in CNS (Central Nerves System). This results in muscle rigidity and spasms (due to the simultaneous contraction of agonists and antagonists, in the absence of reciprocal inhibition Figure 7.11).
Pathogenesis
Clostridium tetani is the causative organism of tetanus or lock jaw disease. pathogenesis of Clostridium tetani was discussed in detail in flowchart 7.4. Source of infection – Soil, dust, faeces. Route of entry – Through wound Incubation period – 6–12 days
Clinical Feature
It includes, pain and tingling at the site of wound, Lock jaw ortrismus (It is reduced opening of the jaws), Risus sardonicus (mouth kept slightly open), Dysphasia (impairment of the ability to speak or to understand language) and acute asphyxia.
Laboratory Diagnosis
Specimens: Wound swab, exudates or tissue from wound.
Myth: Rust causes tetanus if introduced into a wound – for example, by stepping on a rusty nail.
Fact: Rusty nails are more likely to be contaminated with tetanus endospores because they have been exposed to soil and dust longer than new, rust free nails. Any object that causes a wound, rusty or not, can inoculate the tissue with the bacterial endospores of C. tetani. Rust itself neither causes tetanus nor makes it worse.
Microscopy: Gram staining shows Gram positive bacilli with drumstick appearance. Culture: The clinical specimen is inoculated on blood agar and incubated at 37°C for 24–48 hours under anaerobic conditions. The colonies are confirmed by gram staining, where it shows gram positive bacilli with drumstick appearance.
Treatment
Tetanus patients are treated in special isolated units, to protect them from noise and light which may provoke convulsions. The spasm can be controlled by diazepam
Clostridium difficile is the causative agent of antibiotic associated colitis. It is an acute colitis with or without
pseudo membrane formation. It is an important complication in patients on oral antibiotic therapy. Many antibiotics have been incriminated but lincomycin and clindamycin are particularly prone to cause pseudomembranous colitis.
Table 7.9: Immunization for tetanus Active immunization Passive imm
a. Tetanus toxoid (TT) b. DPT
Antitetanus seru Human Antitetan immunoglobulin
Tetanus develops by the contamination of wound with Clostridum tetani spores. The spores germinate in reduced oxygen tension (anaerobic environment).
The toxin blocks synaptic inhibition in the spinal cord. It acts presynaptically.
The toxin affects most of the voluntuary muscles in the body, causing muscle rigidity and spasma due to uncontrolled contractions.
The first symptoms appears in head and neck because of shorter length of cranial nerves. Masseter muscles are first affected causing ‘Lock Jaw’.
The vegetative cell grows and produces a potent neurotoxin called tetanospasmin. The toxin is absorbed from the site of its production and enters into the blood stream. It ascends to central nervous systems (CNS) through motor nerves.
In severe cases progressive spasm of the back or extensor muscles produces opisthotonos (extreme arching of the back) usually death occurs due to respiratory paralysis.
Flowchart 7.4: Pathogenesis of Clostridium tetani
unization Combined prophylaxis m (ATS) us
s (HIIG)
Tetanus toxoid in one arm and ATS or HTIG in another arm.
(0.1–0.2 mg/kg) injection. Antibiotic therapy with penicillin or metronidazole should be done for a week or more.
Prophylaxis
It is done by the following methods, which are as follows. a. Surgical prophylaxis: It aims at
removal of foreign body, blood clots and damaged tissue in order to prevent anaerobic conditions favorable for the germination of spores.
b. Immunoprophylaxis: Tetanus is a preventable disease. Immune prophylaxis is of 3 types, which is given in the (Table 7.9).
Clostridial Toxins As Therapeutic Agents Botulinum toxin is the most poisonous substance known, is being used for the treatment of specific neuromuscular disorders characterized by involuntary muscle contraction. Since approval of Botulinum toxin (botox) by the FDA in 1989 for 3 disorders – Strabismus (crossing of the eyes), blepharospasm (spasmodic contraction of eye muscles) and hemifacial spasm (contraction of one side of the face).
In 2000, dermatologists and plastic surgeons began using Botox to eradicate wrinkles caused by repeated muscle contractions as we laugh, smile or frown.
Infobits
| Ac tiv e imm uniza ti on | Pass iv e imm uniza ti on | C ombine d p rophy l axis |
|---|---|---|
| a. Tet anus t oxoid (T T)b. D PT | Ant itet anus s er um (A T S)Hu ma n Ant ite t anusimm un og lo bu lin s (HII G) | Tet anus t oxoid in o near m a nd AT S o r HTI G inanot her ar m. |
Shigella Dysenteriae (Dysentery Bacillus)
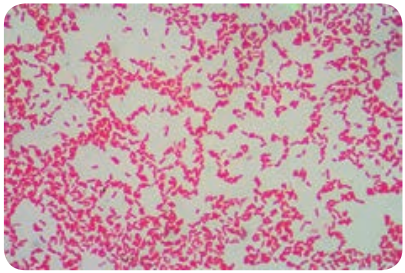
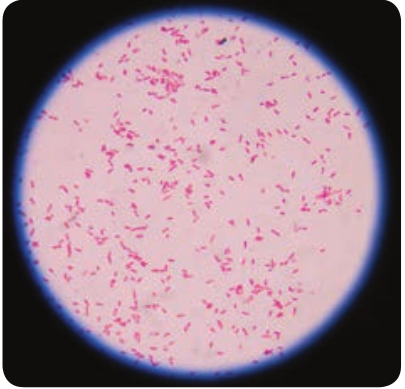
The genus _Shigella_are exclusively parasites of human intestine and other primates. Shigella dysenteriae is the causative agent of bacillary dysentery or shigellosis in humans. It is a diarrheal illness which is characterized by frequent passage of bloodstained mucopurulent stools. The four important species of the genus Shigella are: Shigella dysenteriae, Shigella flexneri, Shigella sonnei and Shigella boydii.
Morphology
Shigella are short, Gram negative rods (0.5µm× 1–3 µm in size). They are non – motile, non – sporing and non – capsulated (Figure 7.12).
Cultural Characteristics
-
They are aerobes and facultative anaerobes. Optimum temperature is 37°C and optimum pH – 7.4.
-
They can be grown on the following media and show the characteristic colony morphology (Table 7.10 & Figure 7.13).
Table 7.10: Colony morphology of Shigella
Media Colony
Morphology Nutrient Agar Colonies are circu-
lar, convex smooth and translucent
MacConkey Agar Colourless colonies
SS – Agar Colourless colonies
Toxins
Shigella dysenteriae produces toxins, which is of 3 types, namely, endotoxin, exotoxin and verocytotoxin. The mode of action of these toxins is illustrated in the Table 7.11.
Pathogenesis
The pathogenic mechanism of _Shigella dysenteriae_is discussed below in flowchart 7.5. Source of Infection – Patient or carriers Route of entry – faecal – oral route
| Me d i a | C o l ony Mor ph ol o g y |
|---|---|
| Nut r ien t A ga r | C olo nies a re cir c u -l ar, co nvex sm o ot hand t ra nslucen t |
| MacC on ke y A ga r | C olo ur les s co lo nies |
| SS – A ga r | C olo ur les s co lo nies |
Site of infection – Large intestine Incubation Period – Less than 48 hours (1–7 days) Mode of transmission – Food, finger, faeces and flies
Clinical Manifestations
-
Frequent passage of loose, scanty faeces containing blood and mucus.
-
Abdominal cramps and tenesmus (straining to defecate).
-
Fever and vomiting.
-
Hemolytic uremic syndrome (It is a condition caused by the abnormal destruction of red blood cells).
Table 7.11: Various toxins of Shigelladysenteriae
Toxins Mode of Action
Endotoxin It is released after autolysis, it has irritating effect on intestinal wall which causes diarrhea and subsequently intestinal ulcers.
Exotoxin It is a powerful toxin and acts as Enterotoxin as well as neurotoxin. As Enterotoxin – It induces fluid accumulation As Neurotoxin – It damages the endothelial cells of small blood vessels of CNS which results in polyneuritis and coma
Vero cytotoxin
It acts on Vero cells
Sh. dysenteriae causes bacillary dysentery. The pathogen enters into the host by the ingestion of contaminated food.
As the pathogen multiples it produces toxins, it stimulates an inflammatory reaction and causes extensive tissue destruction. It leads to necrosis of surface epithelial cells.
The necrotic epithelia, become soft and friable and are sloughed off leaving behind transverse superficial ulcers.
Abdominal cramps and pain are caused by the disruption of the muscular function of the intestine
The degeneration of intestinal villi and local erosion causes bleeding, heavy mucous secretion resulting in bacillary dysentery
The bacilli reaches large intestines and adheres to the epithelial cells of villi. It multiples inside the cell and penetrates into lamina propria.
Flowchart 7.5: Pathogenesis of Shigella dysenteriae
Laboratory Diagnosis
Specimens: Fresh stool is collected. Direct Microscopy: Saline and Lugol’s iodine preparation of faeces show large number of pus cells, and erythrocytes.
| Toxins | Mo de o f Act io n |
|---|---|
| En do toxin | It i s rele as e d af ter a utolysi s,it h as ir r it at in g ef fe c t o nin tes t in a l wal l whic h caus esdi ar rhe a a nd s ubs e quen t lyin tes t in a l u lcer s. |
| E xotoxin | It i s a p ower f u l t oxin a ndac ts a s En ter otoxin a s w el las n eur otoxin.As Ent erot ox i n – It inducesf luid acc um u l at io nAs N e ur otoxin – Itd amages t he en do t heli a lce l ls o f sm a l l b lo o d v es s elsof CNS w hic h r es u lts inp oly neur it is a nd co ma |
| Veroc yt otoxin | It ac ts o n Ver o ce l ls |
Culture: For inoculation, it is best to use mucus flakes (if present in the specimen) on MacConkey agar and SS agar. After overnight incubation at 37°C, the plates are observed for characteristic colonies, which is confirmed by Grams staining and biochemical reactions.
Treatment and Prevention
-
Uncomplicated shigellosis is a self – limiting condition that usually recovers spontaneously.
-
In acute cases, oral rehydration therapy (ORT) is done.
-
In all severe cases, the choice of antibiotic should be based on the sensitivity of prevailing strain.
-
Many strains are sensitive to Nalidixic acid and Norfloxacin.
-
Improving personal and environmental sanitation.
-
The detection and treatment of patients and carriers.
Salmonella Typhi (Eberthella Typhi)
The genus Salmonella consists of bacilli that parasites the intestines of vertebrates and human beings. It causes Enteric fever, which includes Typhoid and Paratyphoid fever. The most important species of the genus is Salmonella typhi which causes typhoid fever.
Morphology
Salmonellae are Gram – negative rods (1– 3µm× 0.5 µm in size). They are motile with peritrichous flagella, non – capsulated and non – sporulated (Figure 7.14).
Cultural Characteristics
They are aerobic and facultative anaerobe, optimum temperature - 37°C and pH is 7–7.5.
They grow on the following media and show the following characteristic colony morphology (Table 7.12).
Table 7.12: Colony morphology of Salmonella typhi
Media Colony Morphology Nutrient Agar
Colonies are large, circular, smooth, translucent
MacConkey Agar
Colourless colonies (non – lactose fermenters)
SS – Agar Colourless colonies with black centered.
Pathogenicity
Salmonella typhi causes typhoid fever and its pathogenesis is discussed in flowchart 7.6. Source of infection – food, feces, fingers, flies
| Me d i a | C ol ony M or ph ol o g y |
|---|---|
| Nut r ien tAg ar | C olo nies are large, circ u l ar,sm o ot h, t ra nslucen t |
| MacC on ke yAg ar | C olo ur les s co lo nies (n on– l ac tos e f er men ter s) |
| SS – A ga r | C olo ur les s co lo nies w it hbl ac k cen ter e d. |
The bacilli reaches the small intestine and attach to the epithelial cells of intestinal villi and penetrate to the lamina propria and submucosa
The infection is acquired by ingestion of contaminated food and water
The pathogen enters the mesenteric lymph nodes, multiply there and enters blood stream through the thoracic duct
A transient bacteraemia follows and internal organs like liver, gall bladder, spleen, bone marrow, lungs, lymph nodes and kidneys are infected
The bacilli multiply abundantly in the gall bladder (bile juice) and are dischared continuously into the intestine involving peyer’s patches and ileum.
These bacilli are phagocytosed by neutophils and macrophages. They resist intracellular killing and multiply within these cells.
It becomes inflamed, necrosed and slough off, leaving behind typhoid ulcers. These ulcers may lead to two major complication, which are Intestinal perforation and haemorrhage.
Flowchart 7.6: Pathogenesis of Salmonella typhi
Route of entry – faecal oralroute (ingestion) Incubation period – 7–14 days
Clinical Manifestations
-
The illness is usually gradual, with headache, malaise (feeling of discomfort), an2orexia (loss of appetite), coated tongue, abdominal discomfort with either constipation or diarrhea.
-
Hepatosplenomegaly (enlargement of liver and spleen), step ladder pyrexia (continuous fever) and rose – spots (during 2nd or 3rd week).
Laboratory Diagnosis
Specimens: Blood, stool and urine are the clinical samples collected from typhoid patients. The selection of relevant specimen depends upon duration of illness, which is very important for diagnosis (Table 7.13 & Figure 7.15).
Table 7.13: Specimen collection for typhoid.
Duration of disease
Specimen examination
% Positivity
1st Week Blood culture 90 2nd Week Blood culture
Faeces culture Widal test
75 50 Low titer
3rd Week Widal test Blood culture Faeces culture
80–100 60 80
| D u r ati on of dis e as e | Sp e cime nexa mina ti on | % Po siti v it y |
|---|---|---|
| 1 Weekst | Blo o d c u ltur e | 90 |
| 2 Weeknd | Blo o d c u ltur eFae ces c u ltur eWid a l t es t | 7550L ow t iter |
| 3 Weekrd | Wid a l t es tBlo o d c u ltur eFae ces c u ltur e | 80–1006080 |
The bacteriological diagnosis of enteric fever consists of the following methods, which are.
- Isolation of the bacill.
- Demonstration of antibodies
Isolation of the bacilli The typhoid bacilli are isolatedfrom the following clinical specimens which are tabulated (Table 7.14). Demonstration of Antibodies: Slide – agglutination: The isolate is identified by slide agglutination with ‘O’ and ‘H’ antisera. Widal Test: It is an agglutination test for detection of agglutinins ‘H’ and ‘O’ in patients with enteric fever. Salmonella antibodies start appearing in the serum at
Table 7.14: Isolation method of typhoid bacill
Specimen culture Blood culture 5–10 ml of
blood cultur or bile broth taurocholate Pale colonies used for mot
Clot Culture (An alternative method to blood culture)
5 ml of bloo allowed to clo rod and adde which digest released from MacConkey a
Faeces culture Faeces sampl agar, DCA or for 24 hours, which is conf
Urine culture Urine sampl inoculated in media.
the end of 1st week and rise sharply during the 3rd week of enteric fever.
Prophylaxis
Various types of vaccine and their doses are given in Table 7.15.
Table 7.15: Various types of vaccine and their doses.
Vaccine Doses TAB – Vaccine
2 doses of 0.5 ml at an interval of 4–6 weeks
Typhoral 3 doses on alternate days. It gives 65–96% protection for 3–5 years and is safe
typhim – Vi
A single dose of 25µg
i from various clinical specimens.
Isolation methods blood is collected and inoculated into e bottle containing taurocholate broth . After overnight incubation at 37°C the broth is subculture on MacConkey agar. (NLF) appear on MacConkey which is
ility and biochemical reactions. d is collected into a sterile test tube and t. The clot is broken up with a sterile glass d to bile broth containing streptokinase, s the clot and there by the bacilli are
the clot. Then it is subcultured on gar.
e are inoculated directly on MacConkey’s SS agar. The plates are incubated at 37°C then characteristic colonies are observed irmed by gram staining. es are centrifuged, and the deposit is to enrichment media and then on selective
| Vac cine | D os es |
|---|---|
| TAB –Vaccin e | 2 dos es of 0.5 ml at an inter va lof 4–6 w e eks |
| Typ hora l | 3 dos es o n a lter nate d ays. I tg ives 65–96% p rote c t io n f or3–5 y e ars a nd i s s afe |
| t yp him –Vi | A sin g le dos e o f 25µg |
| Sp e cime n c u ltur e | Is o l ati on me tho ds |
|---|---|
| Blo o d c u ltur e | 5–10 m l o f b lo o d i s co l le c te d a nd in o c u l ate d in toblo o d c u ltur e b ott le co nt ainin g t aur o chol ate b rot hor b i le b rot h. A f ter o ver nig ht in c ub at io n a t 37°C t het aur o chol ate b rot h i s s ub c u ltur e o n M acC on ke y a ga r.Pa le co lo nies (NLF) a pp e ar o n M acC on ke y w hic h i sus e d f or m ot i li t y a nd b io chemic a l r e ac t io ns. |
| C lo t C u ltur e (A n a lter nat ivemet ho d t o b lo o d c u ltur e) | 5 m l o f b lo o d i s co l le c te d in to a s ter i le t es t t ub e a nda l lo we d to clo t. Th e clo t is broken up wit h a ster i le g l assro d and adde d to bi le brot h co nt ainin g st rep tok in as e,w hic h dig es ts t he c lo t a nd t her e b y t he b aci l li a rerele as e d f rom t he c lo t. Th en i t i s s ub c u ltur e d o nMacC on ke y a ga r. |
| Fae ces c u ltur e | Fae ces s ample a re in o c u l ate d dir e c t ly o n M acC on ke y’saga r, D CA or SS aga r. Th e pl ates are in c ub ate d at 37°Cfor 24 h our s, t hen c harac ter ist ic co lo nies a re o bs er ve dw hic h i s co nf ir me d b y g ra m s t ainin g . |
| Ur in e c u ltur e | Ur in e s amples a re cen t r if ug e d, a nd t he dep osi t i sin o c u l ate d into enric hm en t me di a and t hen on s ele c t iveme di a. |
Treatment and Control Measures
-
Antibacterial therapy has been very effective in the treatment of patients.
-
Ampicillin, amoxicillin and cotrimoxazole are useful in the treatment of typhoid fever.
-
At present, ciprofloxacin is the drug of choice.
-
Typhoid fever can be effectively controlled by sanitary measures for disposal of sewage, clean water supply and supervision of food processing and handling.
Why is proper hand washing considered the most important element in controlling communicable infections?
HOTS
Vibrio Cholerae
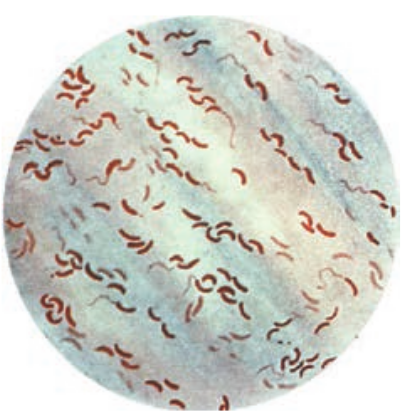
Vibrio is one of the curved rod bacteria, prominent in the Medical Bacteriology. They are present in marine environment and surface waters worldwide. Vibrio is a member of the family Vibrionaceae. The most important member of this genus is Vibrio cholerae, the causative agent of cholera. The term Vibrio is derived from Vibrare (Latin word) which means “to shake or vibrate” and the word Cholera is derived from Chole (Greek word) which means, “to bile” (Figure 7.16).
Morphology
Vibrio cholerae is gram negative, curved or comma shaped, (1.5um x 0.2–0.4um in size) non – capsulated. The organism
is very actively motile with a single polar flagellum and the characteristic movement is called as darting motility. In stained smears of mucus flakes from acute cholera patients, the Vibriois seen arranged in parallel rows. This was described by Robert Koch as “fish in stream” appearance.
Culture Characteristics
Vibrio cholera is strongly aerobic. It grows best in alkaline media with the optimum temperature 37°C and pH 8.2. It is non- halophilic, therefore, cannot grow in media with a concentration of sodium chloride more than 7% (Figure 7.17). Some of the media in which Vibrio cholerae are cultivated are tabulated below in Table 7.16.
Table 7.16: Colony morphology of Vibrio cholerae on various media
Media Colony morphology Nutrient agar The colonies are moist,
translucent round disks (1–2mm in diameter) with a bluish tinge in transmitted light.
MacConkey agar
The colonies are colorless at first but become reddish on prolonged incubation due to late fermentation of lactose.
Thiosulphate citrate bile sucrose agar (pH 8.6)
It is used as a selective medium for isolation of Vibrios. It produces large yellow convex colonies due to sucrose fermentation.
| Me d i a | C ol ony m or ph ol o g y |
|---|---|
| Nut r ien t a ga r | Th e co lo nies a re m oist,t ra nslucen t roun d di sks(1–2mm in di amet er)w it h a b lui sh t in ge int ra nsmi tte d lig ht. |
| MacC on ke yag ar | Th e co lo nies areco lo rles s a t f ir st b utb e co me re ddi sh onprolo nge d in c ub at io ndue to late fer men t at io nof l ac tos e. |
| Thios u lphateci t ra te b i lesucr os e a ga r(pH 8.6) | It i s u s e d a s a s ele c t iveme di um for is ol at io nof Vibr ios . I t p ro ducesl arge yel lo w co nvexco lo nies d ue t o s ucr os efer men t at io n. |
Enterotoxin
Vibrios multiplying on the intestinal epithelium produce an enterotoxin called Cholera toxin. It is also known as Choleragen (or CT). This toxin molecule is approximately 84,000 Dalton and consists of two major subunits namely A and B There is only one subunit in A (1A) whereas there are five subunits in B (5B) (Figure 7.18).
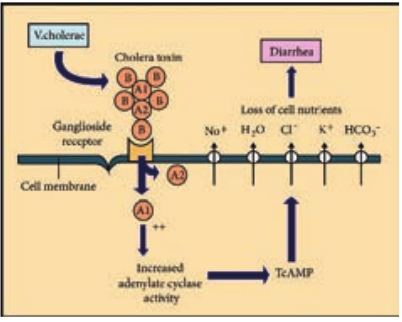
*Mode of Action.
- The B (binding) units of enterotoxin
get attached to the GM1 (Ganglioside membrane receptors I) on the surface of jejunal epithelial cells (target cells).
-
The A (active) subunits then enters the target cell and dissociates into 2 fragments, A1 & A2. The A2 fragment links biologically active A1 fragment to the B – subunit.
-
The A1 fragment causes prolonged activation of cellular adenylate cyclase which in turn accumulates cAMP in the target cell. This leads to outpouring of large quantities of water and electrolytes into small intestinal lumen. Thus, resulting in profuse watery diarrhea.
Natural infection of Vibrio cholerae occurs only in human beings
Pathogenesis
The pathogenic mechanism of Vibrio cholerae is discussed below in flowchart 7.7. Source of Infection – contaminated water or food Route of entry – fecal – oral route Site of infection – small intestine Incubation period – few hours to 5 days (usually 2–3 days)
Vibrio cholerae causes cholera, which is an acute diarrheal disease.
In humans, Vibrio enter orally through contaminated water or food. The ingested pathogens pass through the acid barrier of the stomach & multiply in the small intestine.
In the small intestine, the Vibrio penetrate the mucous barrier & adhere to the microvilli of the epithelial cells & multiply there.
The _Vibrio_is strictly epipathogen& do not penetrate deep into the guts and there is no bacteremia. The virulence of Vibrio cholerae is due to cholera toxin (the mechanism is described earlier in the section 7.10.3 – enterotoxinmode of action)
The toxin also inhibits intestinal absorption of sodium and chloride.
The clinical manifestations & complications are due to massive water and electrolyte depletion.
The voided fluids areodourless and contains flecks of mucus, hence it is known as Rice watery stool
Flowchart 7.7: Pathogenesis of Vibrio cholerae
The loss of water during cholera is about 20–30 litres per day.
Why is cholera the most severe form of gastroenteritis?
HOTS
Clinical Feature
Dehydration, anuria (absence of urine excretion), muscle cramps, hypokalemia (low blood potassium) & metabolic acidosis (low serum concentration of bicarbonates).
Laboratory Diagnosis
Specimen: Stool Direct microscopy: It is not a reliable method for rapid diagnosis, the characteristic darting motility of the vibrio can be observed under dark – field microscope. Culture: Stool sample is directly inoculated on MacConkey agar and TCBS agar. The plates are examined after overnight incubation at 37°C for typical colonies of Vibrio cholera, and the colonies are identified by gram staining and oxidasetest.
Prophylaxis
*1. General Measures.
- Purification of water supplie.
- Improvement of environment
sanitatio.
- Infected patients should be
isolated, and their excreta must be disinfected
2. Vaccines: Two types of oral vaccines have been tried recently.
- Killed oral whole cell vaccine.
- Live oral vaccines
Treatment
1. Oral Rehydrationtherapy: The severe dehydration & salt depletion can be treated by oral rehydration therapy (as recommended by WHO).
2. Antibiotics: It is of secondary importance, oral tetracycline was recommended for reducing the period of Vibrio excretion.
An ideal cholera vaccine is yet to be found.
Mycobacterium Tuberculosis (Tubercle Bacillus)
The genus Mycobacterium is distinguished by its thick, complex, lipidrich waxy cell walls. This high lipid content (Mycolic acids) imparts the characteristic of acid fastness or resistance to decolorizationby a strong acid after staining with carbol fuchsin. Many of the Mycobacterial species are saprophytes but several species are highly significant human pathogens. Mycobacterium tuberculosis is the causative agent of tuberculosis (TB) It is a killer disease and ranks as one of the most serious infection diseases of the developing countries. TB is primarily a disease of the lungs but may spread to other sites of the body.
The name Mycobacterium tuberculosis is derived form,
-
Mycobacterium (Greek) – Fungus like bacterium
-
Tuberculosis (Latin) – Swelling or Knob
Morphology
They are acid fast bacilli, slightly curved rods, it may occur singly or in small clumps. They are non–motile, non–sporing, and non-capsulated.
Cultural Characteristics
They are obligate aerobe, optimum temperature is 37°C and optimum pH is 6.4–7.0. The pathogen grows on an enriched culture media – Lowenstein Jensen medium. The colonies appear in about 2–3 weeks. The colonies are dry, rough, raised, irregular colonies with a wrinkled surface. Initially creamy white and becoming yellowish later (Figure 7.19).
Pathogenesis
Human tuberculosis is divisible into two form, they are Primary TB and Secondary
TB. The pathogenesis of Primary Tuberculosis is described in flowchart 7.8. Source of infection – Airborne droplets Route of entry – Respiratory tract Incubation period – 3–6 weeks.
Non-resident macrophage are also attracted to the site of infection and these macrophages are engulfs the fubercle bacilli. The bacilli were carried through the lymphatic to the local hilar lymph node.
Tubercle bacilli enter the host commonly by inhalation. When bacilli are inhaled, the bacilli enters into the alveolar macrophage, where they can grow and multiply.
Within 10 days of infection, T-lymphocytes produces lymphokines which activate macrophages and leads to form granuloma, around the foci of infection.
The activated marophages are termed epithelioid cells, which fuse to form multinucleate giant cells.
In the lymph nodes, cell mediated immunity (CMI) is stimulated. The CMI response helps to prevent the further spread of pathogen.
The granuloma contains necrotic tissue and dead macrophage which is referred as caseation. Granuloma formation is usually sufficient to limit the primary infection.
The lung lesions is frequently found in the lower lobe and called as ghon focus. The ghon focus together with enlarged hilar lymph nodes is called primary complex.
In some persons, the lesions become dormant and produce dense scar tissue which may become calcified. Some bacilli remain in a dormant form as persisters which, when reactivated, cause post primary TB.
In a minority of cases, the ghon focus ruptures into a blood vessel. Then the bacilli spread throughout the body with the formation of numerous granulomas and known as miliary TB.
Flowchart 7.8: The pathogenesis of Primary Tuberculosis
Secondary TB – (Post primary TB) It is caused by reactivation of the primary lesion or by exogenous reinfection. Granulomas of secondary TB most often occur in the apex of the lungs. The necrotic element of the reaction causes tissue destruction and the formation of large area of caseation termed tuberculomas. The presence of caseous necrosis and cavities are two important clinical manifestations of secondary TB. The cavities may rupture into blood vessels, spreading the bacilli throughout the body and break into airways, releasing the pathogen in aerosols and sputum - called as open tuberculosis (Figure 7.20).
Clinical Symptoms
It includes, cough that lasts for more than 2–3 weeks, weight loss, fever, night sweat and loss of appetite.
M. tuberculosis the world’s most deadly pathogen why?
HOTS
Laboratory Diagnosis
Specimen: In case of pulmonary tuberculosis the most usual specimen is sputum. Direct Microscopy: Smear is made from the sputum specimen and stained by Ziehl – Neelson technique. It is examined under oil immersion objective lens. The acid fast

bacilli appear as bright red bacilli against a blue background.
Culture: The specimen is inoculated onto LJ – medium and incubated at 37°C for 2 weeks the tubercle bacilli usually grow in 2–8 weeks. The bacterial growth is confirmed by Ziehl – Neelsonstaining.
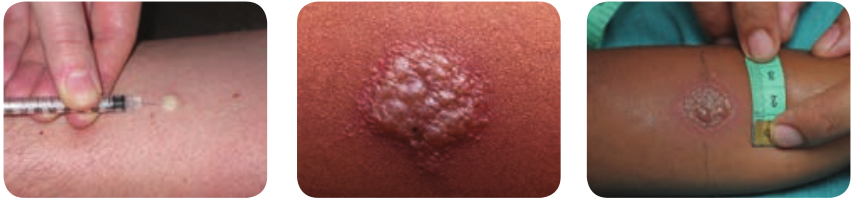
1. Tuberculin Skin test Mantoux test: This method has been used routinely. In this test 0.1 ml of PPD (Purified protein derivative) containing 5 TU (Tuberculin unit) is injected intradermally on the flexor aspect of forearm (Figure 7.21) The site is examined after 48–72 hours and induration are measured (diameter in mm).
Positive test: Indurations of diameter d10 mm or more is considered positive.
Negative test: Indurations of 5 mm or less is negative.
2. Gene Xpert MTB It is an automated diagnosis test it detects DNA sequences specific for M. tuberculosis and rifampicin resistance by PCR. Results can be obtained within 2 hours.
Treatment
The antitubercular drugs include two types of agents which are;
antoux test
Bactericidal agents – Rifampicin (R), Isoniazid (H), Pyrazinamide (z), Streptomycin.
Bacteriostatic agents – Ethambutol (E). The regimen for treating TB consists of an intensive phase of 2 months of isoniazid, rifampin, pyrazinamide and ethambutol, followed by a continuation phase of 4 months of isonizid and Rifampin.
Prophylaxis and Control Measures
The BCG (Bacille – Calmette – Guerin) administered by intradermal injection of the live attenuated vaccine. The immunity may last for about 10 years.
The prevention of TB can be done by the following general measures such as
1. Adequate nutrition. 2. Practicing good hygiene (washing
hands) 3. Health education. 4. Cover the mouth with a tissue when
you cough or sneeze.
Drug Resistance Tuberculosis MDR-TB: Multidrug resistance tuberculosis refers to resistance to rifampian and Isoniazid. MDR-TB is a global problem especially in HIV- patients. XDR-TB: Exensively drug resistance tuberculosis refer M.tuberculosis strains which are resistant to any fluoroquinolone and at lecest one of 3 injectable second line drugs (Kanamycin, Capreomycin and Amikarin), in addition to Isoniazid and rifampicin.
Infobits
24 March is celebrated as World Tuberculosis day
Treponema Pallidum
Treponema pallidum is included in the family Spirochaetaceae. They are slender spirochaetes with fine spirals having pointed ends. Some of them are pathogenic for humans, while others are normal flora in mouth and genitalia. These pathogens are strict parasites and the diseases caused by Treponema are called Treponematoses. Treponema pallidum is the causative agent of syphilis. The name Treponema pallidum is derived from Greek words, which means, Trepos – Turn Nema –Thread and Pallidum – Pale staining.
Morphology
It is thin, delicate spirochete with tapering ends, about 10µm long and 0.1–0.2 µm wide. It has about ten regular spirals, which are sharp and angular, at regular intervals of about 1µm. They are actively motile (endoflagella), exhibiting rotation around the long axis, backward and forward movements and flexion of the whole body. It cannot be seen under light microscope and does not take ordinary bacterial stains. It can be seen under the dark ground (Figure 7.22) or phase contrast microscope. It can be stained by silver impregnation method.
Culture
-
Pathogenic Treponema cannot be grown in artificial culture media.
-
Treponema pallidum strains (Nichol’s strain) are maintained in rabbit testes.
Pathogenesis
Source of infection – Human beings (patients) Mode of transmission – Sexual contact Site of entry – Through minute abrasions/ cuts on skin or mucosa Incubation period – 10–90 days
-
Treponema pallidum causes venereal syphilis, which is acquired by sexual contact. The pathogen enters the human body through cut on the skin or mucosa of genital areas.
-
The clinical disease sets in after an incubation period of about a month. There are 3 clinical stage of venereal syphilis, namely – primary, secondary and tertiary syphilis.
*Primary syphilis.
- A papule appears on the genital area
that ulcerates, forming a chancre of primary syphilis called hard chancre.

-
The chancre is covered by thick exudates, very rich in spirochetes.
-
The regional lymph nodes are swollen, discrete, rubbery and non – tender.
-
Even before the chancre appears, the spirochetes spread from the site of entry into the lymph and bloodstream, so the patient may be infectious during the late incubation period.
-
The chancre invariably heals within 10–40 days, even without treatment, leaving a thin scar.
*Secondary syphilis.
- Secondary syphilis sets in 1–3
months after the primary lesion heals. During this interval, the patient is asymptomatic.
-
The secondary lesions are due to widespread multiplication of the spirochetes and dissemination through the blood.
-
Secondary syphilis is characterized by appearance of papular skin rashes, mucous patches in the oropharynx and condylomata (a raised growth on the skin resembling a wart).
-
The lesions are abundant in spirochetes and the patient is most infectious during the secondary stage.
-
There may be retinitis (inflammation of the retina of the eye), meningitis, periostitis, and arthritis.
-
Secondary lesions usually undergo spontaneous healing, in some cases taking as long as 4 or 5 years.
-
After the secondary lesions disappear, there is a period of dormant known as latent syphilis the patient does not show any clinical symptoms but with positive serology.
*Tertiary syphilis.
- After several years, manifestations
of tertiary syphilis appear. These consist of cardiovascular lesions including aneurysms (enlargement of an artery), gummata (a small rubbery granuloma that has a necrotic centre) and meningovascular manifestations. Tertiary lesions contain few spirochetes.
- In few cases, neurosyphilis such as tabesdorsalis or general paralysis of the insane develops. These are known as late tertiary or quaternary syphilis.
Congenital syphilis In congenital syphilis, the infection is transmitted from mother to fetus by crossing the placental barrier.
Non – Venereal syphilis It may occur in doctors or nurses due to contact with patients lesion during examination. The primary chancre occurs usually on the fingers.
Laboratory Diagnosis
The diagnosis of syphilis includes a. Demonstration of Treponemes b. Serological tests Specimen: Exudates are collected from the chancre. Blood (serum) is collected for serology.
Demonstration of Treponemes a. Dark ground microscopy: The wet film
is prepared with exudates and examined under dark ground microscope. Under dark field examination Treponema pallidum appears motile spiral organism.
b. Treponemes in tissues: It can be demonstrated by silver impregnation method of staining.
Serological tests Non – Treponemal tests – In the standard tests for syphilis includes; a. VDRL – Venereal Diseases Research
Laboratory test. b. RPR – Rapid Plasma Reagain
(Figure 7.23). VDRL or RPR tests are used for
serological screening for syphilis and more useful for the assessment of cure following treatment.
TRUST – Toluidine red unheated serum test, modified form of RPR test.
Treponemal Tests: The treponemal tests includes a. TPHA – Treponema pallidum
hemagglutination assay b. FTA –ABS – Fluorescent treponemal
antibody absorption test. These two tests are used to confirm the
diagnosis.

Treatment and Preventive Measure
In early syphilis a. Benzathine benzyl penicillin,
24 lakhs units intramuscularly in a single dose.
b. Alternatively, doxycycline 100 mg twice a day orally for 15 days.
In late syphilis Benzathine benzyl penicillin 24 lakhs units, intramuscularly once weekly for 3 weeks.
- Avoiding sexual contact with an
infected individual.
- Use of sex barriers (condoms).
Leptospira Interrogans
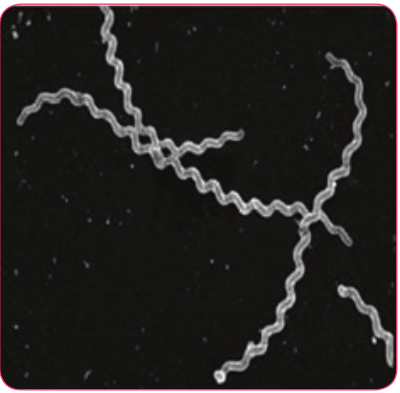
Spirochaetes of the genus Leptospira are actively motile, delicate and possess numerous closely wound spirals with characteristic hooked ends. Several Leptospires are saprophytes, while many are potential pathogens of rodents, domestic animals and humans. The genus Leptospira consists of two important species, which are Leptospira interrogans and Leptospira biflexa.
Leptospira interrrogans is the causative agent of leptospirosis, a zoonotic disease. The word Leptospira is derived from Latin word ‘Leptos’ = fine or thin and ‘spira’ = Coil and interrogans = Question mark (The shape of this spirochete accounts for its name)
Morphology
- They are spiral bacteria (5–20µm × 0.1µm) with numerous closely set coils.
Their ends are hooked and resemble umbrella handles.
-
They are actively motile by rotatory movements.
-
They cannot be seen under light microscope due to its thinness, best observed by dark fieldmicroscopy (Figure 7.24), phase contrast and electron microscope.
-
They stain poorly with aniline dyes, it may be stained with giemsa stain or silver impregnation techniques.
Antigenic Structure
Leptospires show considerable antigenic cross reaction. a. Genus – Specific somatic antigen – It
is present in all members of the genus. b. Surface antigens – This antigen is used
to classify Leptospira into serogroups and serotypes.
Pathogenicity
Source of infection: Contaminated water Route of entry: Through cuts or abrasions on skin or mucosa Incubation period: 6–8 day.
- Leptospira interrogans causes a
zoonotic disease named Leptospirosis. It is transmitted to humans by direct or indirect contact with water, contaminated by urine of carrier animals (rat and dog).
-
Leptospira enter the body through cuts or abrasions on skin or through mucous membranes of the mouth, nose or conjunctiva.
-
After an incubation period of 6–8 days. There is onset of febrile (related to fever) illness with Leptospira in blood (Septicemic phase) which lasts for 3–7 days.
-
The organisms disappear from the blood and invades liver, kidney, spleen, meninges producing meningeal irritation such as headache, vomiting.
-
The pathogen persists in the internal organs and most abundantly in the kidney. Severe Leptospirosis (Weil’s disease) is associated with Fever, conjunctivitis (inflammation of conjunctiva), albuminuria (presence of albumin in the urine), jaundice and hemorrhage. It is a fatal illness with hepatorenal (Kidney failure with severe liver damage).
*Clinical manifestations.
- In severe cases, vomiting, headache,
irregular fever and intense infection of the eyes.
- Jaundice, Albuminuria (The presence of protein Albumin in the urine) and purpuric hemorrhages sometimes occur on skin and mucosa.
Laboratory Diagnosis
The diagnosis of Leptospirosis is made by the following ways
-
Direct microscopy of blood or urine
-
Isolation of pathogen by culture
-
Serological tests.
Direct Microscopy Blood: Leptospira can be observed in the blood by dark – filed microscope. Blood examination is useful in first week as Leptospira disappear from blood after 8 days.
Urine: Leptospira may be present in urine in the 2nd week of the disease and intermittently thereafterup to 6 weeks. Centrifuged deposit of urine may be observed by Dark filed microscopy.
Culture: Blood (1st week) and urine (2nd–6 week) can be cultured in Korthof ’s medium. Media are incubated at 37°C for 2 days and then left at room temperature for 2 weeks. Culturesare examined every third day for the presence of Leptospira under DFM.
Serological tests It is very useful method of diagnosis two types of serological tests are used, which are,
a. Screening tests: These tests are genus – specific and done using reactive genus specific antigen (non – pathogenic L. biflexapatoc I strain).
Screening test includes – CFT, ELISA, SEL, HAT indirect IF these tests are capable to detect IgM and IgG leptospiral antibodies.
b. Serotype specific tests: These tests identify the infecting serovar by demonstrating specific antibodies. i. Macroscopic agglutination test
ii. Microscopic agglutination test
Treatment and Preventions
Leptospira are sensitive to penicillin and tetracycline
Preventive measures include rodent control, disinfection of water.
Summary
Pathogenicity refers to the ability of a pathogen to produce disease. Virulence is the ability of the pathogen to cause disease. The various bacterial pathogens, its pathogenesis clinical symptoms, laboratory diagnosis, control, prophylaxis and treatment with appropriate antibiotics are discussed below. S. aureus
is a leading cause of hospital acquired infections. Cloxacillin is used against beta lactamase. Producing strains Streptococcus pyogenes is intrinsically a much more dangerous pathogen than Staphylococcus aureus and has a much greater tendency to spread in the tissues. Streptococcal pyrogenic exotoxin leads to streptococcal toxic shock syndrome (TSS). A common cross – reacting antigen exist in some group A streptococci and heart, therefore, antibodies produced in response to the streptococcal infection could cross react with myocardial and heart valve tissue, causing cellular destruction. N. meningitidis is the causative agent of meningococcal meningitis, also known as pyogenic or septic meningitis. Clostridium tetani is the causative organism of tetanus or lock jaw disease. The four important species of the genus Shigella are: Shigella dysenteriae, Shigella flexneri, Shigella sonnei and Shigella boydii. Several Leptospires are saprophytes, while many are potential pathogens of rodents, domestic animals and humans.
Evaluation
Multiple choice questions
1. Which of the following toxin is responsible for staphylococcal scalded skin syndrome? a. Epidermolytic
toxin b. Enterotoxin c. Coagulase d. Haemolysin
2. The most important bacterial cause of sore throat is a. Staphylococcus aureaus b. Streptococcus pyogenes c. Haemophilus influenzae d. Escherichia coli
3. The causative agent of waterhouse – friderichsen syndrome is a. Neisseria meningitidis b. Streptococcus pyogenes c. Treponema pallidum d. Staphylococcus aureus
4. A gram-positive bacilli possessing metachromatic granules, showing Chinese letters arrangement are characteristic of a. Corynebacterium diphtheriae b. Mycobacterium tuberculosis c. Bacillus anthracis d. Clastridium perfringens
5. Drumstick appearance of spores is a characteristic feature of a. Clostridium difficle b. Clostridium perfringens c. Clostridium botulinum d. Clostridium tetani
6. Shigella dysenteriae causes a. Bacillary dysentery b. amoebic dysentery c. Traveller’s diarrhoea d. Colitis
7. The most important specimen for isolation of Salmonella typhi in the first week of enteric fever is a. Blood b. Urine c. CSF d. Faeces
8. Which of the following methods can be used to demonstrate T. pallidum? a. Silver impregnation method b. Dark ground microscopy c. Immuno fluorescence staining d. All of the above
9. Weil’s disease is caused by which of the following bacteria a. Salmonella typhi b. Escherichia coli c. Leptospira interrogans d. Vibrio cholerae
10. BCG Vaccine is a. Live attenuated vaccine b. Toxoid c. Killed vaccine d. None of the above
Answer the following
1. Enlist the toxins and enzymes produced by Staphylococcus aureus.
2. Write a short note on non-suppurative complications of streptococcus pyogenes infections.
3. Write about Meningococcal vaccines. 4. Write the prophylaxis for diphtheria
5. Enlist the difference between bacillary dysentery and amoebic dysentery/
6. What is enteric fever? 7. Write the prophylaxis for Salmonella
typhi. 8. Write the mode of action of cholera
toxin/ 9. Tubercubin skin test.
10. Define chancre. 11. Write the morphology of Clostridium
tetani 12. List out the antituberculosis drugs. 13. Write the colony morphology of
salmonella typhi shigella dynsenteriae on ss-agar.
14. Discuss the pathogenesis of typhoid fever.
15. Describe the pathogenesis of pulmonary tuber culosis.
16. Write briefly about Lab/diagnosis of tuberculosis.
17. Explain the pathogenesis of V. Cholerae
18. Write the mode of action of tetanospasmin
19. Describe the pathogenesis of lock-jaw disease.
20. What is staphylococcal toxic shock syndrome (STSS).
21. Describe the mode of entry of Meningococci from nusopharymx to brain.
22. Why antibiotics are avoided in dynsentery except in severe cases.
23. Define zoonotic bacterial disease and give two examples.
24. Describe the pathogenesis of primary syphilis.
25. Enlist the recent serologtical methods used to diagnose tuberculosis.
26. Write the colony morphology of Lowenstein – Jensen.