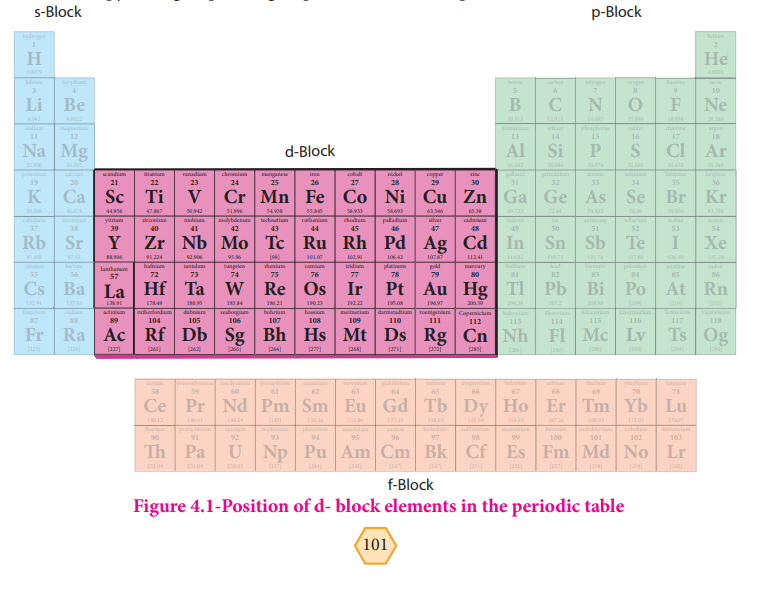
Position of d- block elements in the periodic table:
We have already learnt the periodic classification of elements in XI std. the transition
metals occupy from group –3 to group-12 of the modern periodic table.
nitrogen
14.007 N
7
helium
He 4.0026
2
neon
Ne 20.180
10 uorine
F 18.998
9 oxygen
O 15.999
8 carbon
C 12.011
6 boron
B 10.811
5
argon
Ar 39.948
18 chlorine
Cl 35.453
17 sulfur
S 32.065
16 phosphorus
P 30.974
15 silicon
Si 28.086
14 aluminium
Al 26.982
13
krypton
Kr 83.798
36 bromine
Br 79.904
35 selenium
Se 78.96
34 arsenic
As 74.922
33 germanium
Ge 72.64
32 gallium
Ga 69.723
31 zinc
Zn 65.38
30 copper
Cu 63.546
29 nickel
Ni 58.693
28 cobalt
Co 58.933
27 iron
Fe 55.845
26 manganese
Mn 54.938
25 chromium
Cr 51.996
24 vanadium
V 50.942
23 titanium
Ti 47.867
22 scandium
Sc 44.956
21 calcium
Ca 40.078
20 potassium
K 39.098
19
magnesium
Mg 24.305
12 sodium
Na 22.990
11
beryllium
Be 9.0122
4 lithium
Li 6.941
3
hydrogen
H 1.0079
1
xenon
Xe 131.29
54 iodine
I 126.90
53 tellurium
Te 127.60
52 antimony
Sb 121.76
51 tin
Sn 118.71
50 indium
In 114.82
49 cadmium
Cd 112.41
48 silver
Ag 107.87
47 palladium
Pd 106.42
46 rhodium
Rh 102.91
45 ruthenium
Ru 101.07
44 technetium
Tc [98]
43 molybdenum
Mo 95.96
42 niobium
Nb 92.906
41 zirconium
Zr 91.224
40 yttrium
Y 88.906
39 strontium
Sr 87.62
38 rubidium
Rb 85.468
37
radon
Rn [222]
86 astatine
At [210]
85 polonium
Po [209]
84 bismuth
Bi 208.98
83 lead
Pb 207.2
82
dysprosium
Dy 162.50
66 terbium
Tb 158.93
65 gadolinium
Gd 157.25
64 europium
Eu 151.96
63 samarium
Sm 150.36
62 promethium
Pm [145]
61 neodymium
Nd 144.24
60 praseodymium
Pr 140.91
59 cerium
Ce 140.12
58
lanthanum
La 138.91
57 barium
Ba 137.33
56 caesium
Cs 132.91
55
roentgenium
Rg [272]
111 Copernicium
Cn [285]
112 Nahonium
Nh [286]
113 Flerovium
Fl [289]
114 Mascovium
Mc [289]
115 Livermorium
Lv [293]
116 Tennessine
Ts [294]
117 Oganessom
Og [294]
118 darmstadtium
Ds [271]
110 meitnerium
Mt [268]
109 hassium
Hs [277]
108 bohrium
Bh [264]
107 seaborgium
Sg [266]
106 dubnium
Db [262]
105 rutherfordium
Rf [261]
104 radium
Ra [226]
88 francium
Fr [223]
87
lutetium
Lu 174.97
71 ytterbium
Yb 173.05
70 thulium
Tm 168.93
69 erbium
Er 167.26
68 holmium
Ho 164.93
67
thallium
Tl 204.38
81 mercury
Hg 200.59
80 gold
Au 196.97
79 platinum
Pt 195.08
78 iridium
Ir 192.22
77 osmium
Os 190.23
76 rhenium
Re 186.21
75 tungsten
W 183.84
74 tantalum
Ta 180.95
73 hafnium
Hf 178.49
72
berkelium
Bk [247]
97 lawrencium
Lr [262]
103 nobelium
No [259]
102 mendelevium
Md [258]
101 fermium
Fm [257]
100 einsteinium
Es [252]
99 californium
Cf [251]
98 curium
Cm [247]
96 americium
Am [243]
95 plutonium
Pu [244]
94 neptunium
Np [237]
93 uranium
U 238.03
92 protactinium
Pa 231.04
91 thorium
232.04
90
actinium
Ac [227]
89
s-Block
d-Block
p-Block
f-Block Figure 4.1-Position of d- block elements in the periodic table
XII U4-D-Block-Jerald Folder.indd 101 2/19/2020 4:40:17 PM
| L BN MC d-Block6.94 9.012 10.81 12.011 14.00sod1ium magnesium1 aluminium silico phosphorus22.99 24.30pota1ssium calci2 um scandium titanium vanadium chromium manganese iron cobalt nickel copper zinc39.09 40.07rubidium strontium | N9 115.99 18.99 20.18sulfur chlorin argon | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A126.98gallium | S P14 128.08 30.97germanium arsenic | 132.06selenium | Cl Ar1 135.45 39.94bromin krypto | |||||||||||||
| Sc Ti V Cr Mn Fe Co Ni Cu ZnY Zr Nb Mo Tc Ru Rh Pd Ag CdLa21 Hf22 Ta23 W24 Re25 Os26 Ir27 Pt28 Au29 Hg3044.956 47.867 50.942 51.996 54.938 55.845 58.933 58.693 63.546 65.38Acyttr39ium zirRfco40nium Dbnio41bium molySg42bdenum teBhchne43tium ruHstheni44 um Mtrhod45ium palladiDs46um Rgsilver47 Cncadmi48um88.906 91.224 92.906 95.96 [98] 101.07 102.91 106.42 107.87 112.41lant57hanum hafn 72ium tant 73alum tungsten74 rhenium75 osmi76um irid77ium plat 78inum gold79 merc80ury138.91 178.49 180.95 183.84 186.21 190.23 192.22 195.08 196.97 200.59actinium89 ruther 104fordium dubn105ium seab 106orgium bo107hrium hassium108 meitnerium109 darm 110stadtium roen 111tgenium Copernici112 um[227] [261] [262] [266] [264] [277] [268] [271] [272] [285] | G369.72indium | G372.6ti | As S Br3 34 374.92 78.9 79.90antimony tellurium iodin | Kr383.79xeno | ||||||||||||
| Rb Sr3 385.46 87.6caesium bariu | In49114.82thallium | Sn5118.71lead | S5121.7bismut | T5127.6polonium | 5126.90astatine | X54131.2rado | ||||||||||
| C5132.91francium | B5137.3radium | T81204.3Nahoniu | P B Po A82 8 84 8207.2 208.98 [209 [210Flerovium Mascovium Livermorium Tennessine | Rn8[222Oganessom | ||||||||||||
| Fr8[223 | R8[226 | |||||||||||||||
| N113[286] | F114[289] | Mc11[289 | L11[293 | Ts11[294 | Og11[294 |
| Pr N P Sm EPa U Np59 60 61 62 6140.91 144.24 [145] 150.3 151.96protactiniu91 urani92um neptunium9 plutonium americiu231.04 238.03 [237] | G T Dy6 6 6157.2 158.93 162.5curium berkelium californiu | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H Er T6 68 69164.93 167.2 168.93einsteiniu fermium mendeleviu | Y70173.0nobelium | ||||||||
| P94[244] | Am95[243] | C B9 9[247] [247] | Cf Es98 99[251] [252] | F M No100 101 102[257] [258] [259] |
d- Block elements composed of 3d series (4th period) Scandium to Zinc ( 10 elements), 4d series ( 5th period) Yttrium to Cadmium ( 10 elements) and 5d series ( 6th period) Lanthanum, Haffinium to mercury. As we know that the group-12 elements Zinc, Cadmium and Mercury do not have partially filled d-orbital either in their elemental state or in their normal oxidation states. However they are treated as transition elements, because their properties are an extension of the properties of the respective transition elements. As per the IUPAC definition, the seventh period elements, starting from Ac, Rf to Cn also belong to transition metals. All of them are radioactive. Except Actinium; all the remaining elements are synthetically prepared and have very low half life periods.