important compound of Transition elements
Oxides and Oxoanions of Metals
Generally, transition metal oxides are formed by the reaction of transition metals with molecular oxygen at high temperatures. Except the first member of 3d series, Scandium, all other transition elements form ionic metal oxides. The oxidation number of metal in metal oxides ranges from +2 to +7. As the oxidation number of a metal increases, ionic character decreases, for example, Mn2O7 is covalent. Mostly higher oxides are acidic in nature, Mn2O7 dissolves in water to give permanganic acid (HMnO4 ) , similarly CrO3 gives chromic acid (H2CrO4) and dichromic acid (H2Cr2O7). Generally lower oxides may be amphoteric or basic, for example, Chromium (III) oxide - Cr2O3, is amphoteric and Chromium(II) oxide, CrO, is basic in nature.
Potassium dichromate \(\text{K}_2\text{Cr}_2\text{O}_7\) :
Preparation:
Potassium dichromate is prepared from chromate ore. The ore is concentrated by gravity separation. It is then mixed with excess sodium carbonate and lime and roasted in a reverbratory furnace.
\[4 \text{FeCr}_2\text(O)_4 + 8 \text{Na}_2\text{CO}_3 +7 \text{O}_2 \xrightarrow[900-1000AC]{}8 \text{Na}_2\text{CrO}_4 + 2 \text{Fe}_2 \text{O}_3 +8 \text{CO}_2 \uparrow\]The roasted mass is treated with water to separate soluble sodium chromate from insoluble iron oxide. The yellow solution of sodium chromate is treated with concentrated sulphuric acid which converts sodium chromate into sodium dichromate.
\[ \underset{\text{sodium chromate (yellow)}}{ 2 \text{Na}_2\text{CrO}_4} + \text{H}_2\text{SO}_4 \rightarrow \underset{\text{sodium dichromate (orange red)}}{\text{Na}_2\text{Cr}_2\text{O}_7} + 2 \text{Na}_2\text{SO}_4 + \text(H)_2\text{O} \]The above solution is concentrated to remove less soluble sodium sulphate. The resulting solution is filtered and further concentrated. It is cooled to get the crystals of Na2SO4.2H2O.
The saturated solution of sodium dichromate in water is mixed with KCl and then concentrated to get crystals of NaCl. It is filtered while hot and the filtrate is cooled to obtain \(\text{K}_2\text{Cr}_2\text{O}_7\) crystals.
\[ \underset{\text{sodium dichromate (orange red}}{ \text{Na}_2\text{Cr}_2\text{O}_7} + 2 \text{K}\text{Cl} \rightarrow \underset{\text{potassium dichromate (orange red)}}{\text{k2}_2\text{Cr}_2\text{O}_7} + 2 \text{Na}\text{Cl}_4 \]Physical properties:
Potassium dichromate is an orange red crystalline solid which melts at 671K and it is moderately soluble in cold water, but very much soluble in hot water. On heating it decomposes and forms Cr2O3 and molecular oxygen. As it emits toxic chromium fumes upon heating, it is mainly replaced by sodium dichromate.
\[4 \text{K}_2\text(Cr)_2\text{O}_7 \xrightarrow{\overset{\bigtriangleup}{}}4 \text{K}_2\text{CrO}_4 + 2 \text{Cr}_2 \text{O}_3 +3 \text{CO}_2 \uparrow\]Structure of dichromate ion:
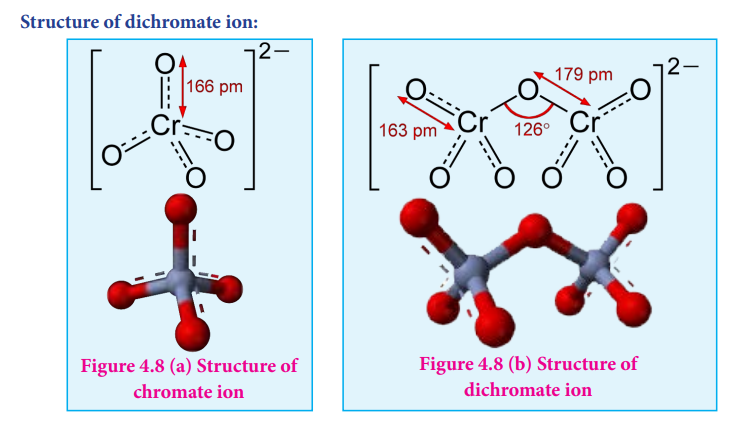
Both chromate and dichromate ion are oxo anions of chromium and they are moderately
strong oxidizing agents. In these ions chromium is in +6 oxidation state. In an aqueous solution, chromate and dichromate ions can be interconvertible, and in an alkaline solution chromate ion is predominant, whereas dichromate ion becomes predominant in acidic solutions. Structures of these ions are shown in the fi gure.
Chemical properties:
1. Oxidation
Potassium dichromate is a powerful oxidising agent in acidic medium. Its oxidising action in the presence of H+ ions is shown below. You can note that the change in the oxidation state of chromium from Cr6+ to Cr3+.Its oxidising action is shown below. Cr O + 14H + 6e r + 7 H O2 7 \[\]
2 +
3+ 2
− − → 2C
Th e oxidising nature of potassium dichromate (dichromate ion) is illustrated in the following examples.
Figure 4.8 (a) Structure of chromate ion

Figure 4.8 (b) Structure of dichromate ion
XII U4-D-Block-Jerald Folder.indd 113 2/19/2020 4:41:02 PM
(i) It oxidises ferrous salts to ferric salts.
Cr O + 6Fe + 14H 2Cr + 6Fe + 7H O2 7 2 2+
3+ 3+
2 − + →
(ii) It oxidises iodide ions to iodine
Cr O + 6I + 14H 2Cr + 3I + 7H O2 7 2
3+
2 2 − +−
→
(iii) It oxidises sulphide ion to sulphur
Cr O + 3S + 14H 2Cr + 3S + 7H O2 7 2
3+
2 − +−
→ 2
(iv) It oxidises sulphur dioxide to sulphate ion
Cr O + 3SO + 2H 2Cr + 3SO + H O2 7 2
3+
4 2
2 − + −→2
(v) It oxidises stannous salts to stannic salt
Cr O + 3Sn + 14H 2Cr + 3Sn + 7H O2 7 2
3+
2 − ++ +
→ 2 4
(vi) It oxidises alcohols to acids.
2K Cr O + 8H SO + CH CH OH 2K SO + 2Cr SO + 3C2 2 7 2 4 3 2
2 4 2 4 3
3 → ( ) H COOH + 11H O3 2
2. Chromyl chloride test:
When potassium dichromate is heated with any chloride salt in the presence of Conc H2SO4, orange red vapours of chromyl chloride (CrO2Cl2) is evolved. This reaction is used to confirm the presence of chloride ion in inorganic qualitative analysis.
K Cr O + 4NaCl + H SO 2KHSO + 4NaHSO + 2CrO Cl 2 2 7 2 4 4 4 2 2
C 6 →
hromyl chloride 2+ 3H O↑
The chromyl chloride vapours are dissolved in sodium hydroxide solution and then acidified with acetic acid and treated with lead acetate. A yellow precipitate of lead chromate is obtained.
CrO Cl + 4NaOH Na CrO + 2NaCl + 2H O Na CrO + CH
2 2 2 4 2
2 4 3
→ COO Pb PbCrO + 2C
2 4( ) → ↓
Leadchromate Yellowprecipitate( )
H COONa3
Uses of potassium dichromate:
Some important uses of potassium dichromate are listed below.
1. It is used as a strong oxidizing agent.
2. It is used in dyeing and printing.
3. It used in leather tanneries for chrome tanning.
4. It is used in quantitative analysis for the estimation of iron compounds and iodides.
XII U4-D-Block-Jerald Folder.indd 114 2/19/2020 4:41:09 PM
Potassium permanganate - KMnO4
Preparation:
Potassium permanganate is prepared from pyrolusite (MnO2) ore. The preparation involves the following steps.
(i) Conversion of MnO2 to potassium manganate: Powdered ore is fused with KOH in the presence of air or oxidising agents like KNO3 or
KClO3. A green coloured potassium manganate is formed.
2MnO + 4KOH + O K MnO
+ 22 2 4→ potassium manganate
Green( )
2H O22
(ii) Oxidation of potassium manganate to potassium permanganate: Potassium manganate thus obtained can be oxidised in two ways , either by chemical
oxidation or electrolytic oxidation.
Chemical oxidation:
In this method potassium manganate is treated with ozone (O3) or chlorine to get potassium permanganate.
2MnO + O + H O 2MnO + 2OH + O 2MnO + C
4 2
3 2 4 2
4 2
− − −
−
→ l 2MnO + 2Cl2
4→ − −
Electrolytic oxidation
In this method aqueous solution of potassium manganate is electrolyzed in the presence of little alkali.
K MnO 2K + MnO
H O H + OH 2 4
+ 4
2 +
⇀ ↽ ⇀ ↽
2−
−
Manganate ions are converted into permanganate ions at anode.
Green purple MnO MnO + e4 4
2 −− −2 2 2
H2is liberated at the cathode.
2H + 2e H + 2
− → ↑
The purple coloured solution is concentrated by evaporation and forms crystals of potassium permanganate on cooling.
Physical properties:
Potassium permanganate exists in the form of dark purple crystals which melts at 513 K. It is sparingly soluble in cold water but, fairly soluble in hot water.
XII U4-D-Block-Jerald Folder.indd 115 2/19/2020 4:41:16 PM
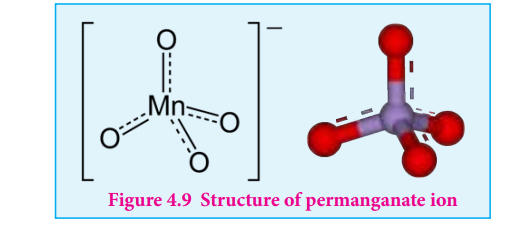
Structure of permanganate ion
Permanganate ion has tetrahedral geometry in which the central Mn7+ is sp3 hybridised.
Chemical properties:
1. Action of heat:
When heated, potassium permanganate decomposes to form potassium manganate and manganese dioxide.
2KMnO 2K MnO + MnO + O4 2 4 2 2→
2. Action of conc H2SO4
On treating with cold conc H2SO4, it decomposes to form manganese heptoxide, which subsequently decomposes explosively.
2KMnO + 2H SO (cold)
Mn O + 2KHSO + H O
Mn O
4 2 4 2 7 4 2
2 7
→
→∆ 4MnO + 3O2 22
But with hot conc H2SO4, potassium permanganate give MnSO4
4KMnO + 6H SO (hot)
4MnSO + 2K SO + 6H O + 5O4 2 4 4 2 4 2 2→
3. Oxidising property:
Potassium permanganate is a strong oxidising agent, its oxidising action diff ers in diff erent reaction medium.
a) In neutral medium: In neutral medium, it is reduced to MnO2
.
MnO + 2H O + 3e MnO + 4OH4 2 2
− − −→
(i) It oxidises H2S to sulphur
2MnO + 3H S 2MnO + 3S + 2OH + 2H O4 2 2 2
− −→
(ii) It oxidises thiosulphate into sulphate
8MnO + 3S O + H O 6SO + 8MnO + 2OH4 2 3 2
2 4 2
− − − −→ 2
Figure 4.9 Structure of permanganate ion
XII U4-D-Block-Jerald Folder.indd 116 2/19/2020 4:41:19 PM
b) In alkaline medium: In the presence of alkali metal hydroxides, the permanganate ion is converted into
manganate.
MnO + e MnO 4 4
− − −→ 2
This manganate is further reduced to MnO2 by some reducing agents.
MnO + H O MnO + 2OH + O4 2 2
2− −→ [ ] So the overall reaction can be written as follows. MnO + 2H O + 3e MnO + 4OH4 2
− − −→
This reaction is similar as that for neutral medium.
Bayer’s reagent:
Cold dilute alkaline KMnO4 is known as Bayer’s reagent. It is used to oxidise alkenes into diols. For example, ethylene can be converted into ethylene glycol and this reaction is used as a test for unsaturation.
c) In acid medium:
In the presence of dilute sulphuric acid, potassium permanganate acts as a very strong oxidising agent. Permanganate ion is converted into Mn2+ ion.
MnO + 8H + 5e Mn + 4H O4 +
2+
2 − − →
The oxidising nature of potassium permanganate (permanganate ion) in acid medium is illustrated in the following examples.
(i) It oxidises ferrous salts to ferric salts.
2MnO + 10Fe + 16H 2Mn + 10Fe + 8H O4 2+
2+ 3+
2 − + →
(ii) It oxidises iodide ions to iodine
2MnO + 10 I + 16H 2Mn + 5I + 8H O4
2+ 2 2
− − + →
(iii) It oxidises oxalic acid to CO2
2MnO + COO + 16H 2Mn + 10CO + 8H O4
2+ 2 2
− − +( ) →5 2
(iv) It oxidises sulphide ion to sulphur
2MnO + S + 16H 2Mn + 5 S + 8H O4 2
2+
2 − − + →5
(v) It oxidises nitrites to nitrates
2MnO + 5NO + 6H 2Mn + 5NO + 3H O4 2
2+ 3 2
− − + −→
(vi) It oxidises alcohols to aldehydes.
2KMnO + 3H SO + CH CH OH K SO + 2MnSO + 5CH CHO 4 2 4 3 2 2 4 4 35 → + 8H O2
(vii) It oxidises sulphite to sulphate
2MnO + 5SO + 6H 2Mn + 5SO + 3H O4 3
2+ 4 2
− − + −→2 2
XII U4-D-Block-Jerald Folder.indd 117 2/19/2020 4:41:29 PM
Uses of potassium permanganate:
Some important uses of potassium permanganate are listed below.
1. It is used as a strong oxidizing agent.
2. It is used for the treatment of various skin infections and fungal infections of the foot.
3. It used in water treatment industries to remove iron and hydrogen sulphide from well water.
4. It is used as Bayer’s reagent for detecting unsaturation in an organic compound.
5. It is used in quantitative analysis for the estimation of ferrous salts, oxalates, hydrogen peroxide and iodides.
Note HCl cannot be used for making the medium acidic since it reacts with KMnO4 as follows.
2MnO + 10 Cl + 16H 2Mn + 5Cl + 8H O4
2+ 2 2
− − + →
HNO3 also cannot be used since it is good oxidising agent and reacts with reducing agents in the reaction.
However,H2SO4 is found to be most suitable since it does not react with potassium permanganate.
Equivalent weight of KMnO4 in acid medium =
Molecular weight of KMnO no of mols of electrons transf
erred = 158
5 = 31.6
Equivalent weight of KMnO4 in basic medium =
Molecular weight of KMnO no of mols of electrons transfe
rred = 158
1 = 158
Equivalent weight of KMnO4 in neutral medium =
Molecular weight of KMnO no of mols of electrons transfe
rred = 158
3 = 52.67
f-block elements – Inner transition elements
In the inner transition elements there are two series of elements.
-
Lanthanoids ( previously called lanthanides)
-
Actinoids ( previously called actinides)
Lanthanoid series consists of fourteen elements from Cerium (58Ce) to Lutetium (71Lu) following Lanthanum (57La).These elements are characterised by the preferential filling of 4f orbitals, Similarly actinoids consists of 14 elements from Thorium (90Th) to Lawrencium (103Lr) following Actinium (89Ac).These elements are characterised by the preferential filling of 5f orbital.
The position of Lanthanoids in the periodic table
The actual position of Lanthanoids in the periodic table is at group number 3 and period
Note
XII U4-D-Block-Jerald Folder.indd 118 2/19/2020 4:41:33 PM
number 6.However, in the sixth period after lanthanum, the electrons are preferentially filled in inner 4f sub shell and these fourteen elements following lanthanum show similar chemical properties. Therefore these elements are grouped together and placed at the bottom of the periodic table. This position can be justified as follows.
1. Lanthanoids have general electronic configuration [Xe] f d s4 5 61 14 0 1 2− −
2. The common oxidation state of lanthanoides is +3
3. All these elements have similar physical and chemical properties.
Similarly the fourteen elements following actinium resemble in their physical and chemical properties. If we place these elements after Lanthanum in the periodic table below 4d series, the properties of the elements belongs to a group would be different and it would affect the proper structure of the periodic table. Hence a separate position is provided to the inner transition elements as shown in the figure.
nitrogen
14.007 N
7
helium
He 4.0026
2
neon
Ne 20.180
10 uorine
F 18.998
9 oxygen
O 15.999
8 carbon
C 12.011
6 boron
B 10.811
5
argon
Ar 39.948
18 chlorine
Cl 35.453
17 sulfur
S 32.065
16 phosphorus
P 30.974
15 silicon
Si 28.086
14 aluminium
Al 26.982
13
krypton
Kr 83.798
36 bromine
Br 79.904
35 selenium
Se 78.96
34 arsenic
As 74.922
33 germanium
Ge 72.64
32 gallium
Ga 69.723
31 zinc
Zn 65.38
30 copper
Cu 63.546
29 nickel
Ni 58.693
28 cobalt
Co 58.933
27 iron
Fe 55.845
26 manganese
Mn 54.938
25 chromium
Cr 51.996
24 vanadium
V 50.942
23 titanium
Ti 47.867
22 scandium
Sc 44.956
21 calcium
Ca 40.078
20 potassium
K 39.098
19
magnesium
Mg 24.305
12 sodium
Na 22.990
11
beryllium
Be 9.0122
4 lithium
Li 6.941
3
hydrogen
H 1.0079
1
xenon
Xe 131.29
54 iodine
I 126.90
53 tellurium
Te 127.60
52 antimony
Sb 121.76
51 tin
Sn 118.71
50 indium
In 114.82
49 cadmium
Cd 112.41
48 silver
Ag 107.87
47 palladium
Pd 106.42
46 rhodium
Rh 102.91
45 ruthenium
Ru 101.07
44 technetium
Tc [98]
43 molybdenum
Mo 95.96
42 niobium
Nb 92.906
41 zirconium
Zr 91.224
40 yttrium
Y 88.906
39 strontium
Sr 87.62
38 rubidium
Rb 85.468
37
radon
Rn [222]
86 astatine
At [210]
85 polonium
Po [209]
84 bismuth
Bi 208.98
83 lead
Pb 207.2
82
dysprosium
Dy 162.50
66 terbium
Tb 158.93
65 gadolinium
Gd 157.25
64 europium
Eu 151.96
63 samarium
Sm 150.36
62 promethium
Pm [145]
61 neodymium
Nd 144.24
60 praseodymium
Pr 140.91
59 cerium
Ce 140.12
58
lanthanum
La 138.91
57 barium
Ba 137.33
56 caesium
Cs 132.91
55
roentgenium
Rg [272]
111 Copernicium
Cn [285]
112 Nahonium
Nh [286]
113 Flerovium
Fl [289]
114 Mascovium
Mc [289]
115 Livermorium
Lv [293]
116 Tennessine
Ts [294]
117 Oganessom
Og [294]
118 darmstadtium
Ds [271]
110 meitnerium
Mt [268]
109 hassium
Hs [277]
108 bohrium
Bh [264]
107 seaborgium
Sg [266]
106 dubnium
Db [262]
105 rutherfordium
Rf [261]
104 radium
Ra [226]
88 francium
Fr [223]
87
lutetium
Lu 174.97
71 ytterbium
Yb 173.05
70 thulium
Tm 168.93
69 erbium
Er 167.26
68 holmium
Ho 164.93
67
thallium
Tl 204.38
81 mercury
Hg 200.59
80 gold
Au 196.97
79 platinum
Pt 195.08
78 iridium
Ir 192.22
77 osmium
Os 190.23
76 rhenium
Re 186.21
75 tungsten
W 183.84
74 tantalum
Ta 180.95
73 hafnium
Hf 178.49
72
berkelium
Bk [247]
97 lawrencium
Lr [262]
103 nobelium
No [259]
102 mendelevium
Md [258]
101 fermium
Fm [257]
100 einsteinium
Es [252]
99 californium
Cf [251]
98 curium
Cm [247]
96 americium
Am [243]
95 plutonium
Pu [244]
94 neptunium
Np [237]
93 uranium
U 238.03
92 protactinium
Pa 231.04
91 thorium
232.04
90
actinium
Ac [227]
89
s-Block
d-Block
p-Block
f-Block
Electronic configuration of Lanthanoids:
We know that the electrons are filled in different orbitals in the order of their increasing energy in accordance with Aufbau principle. As per this rule after filling 5s,5p and 6s and 4f level begin to fill from lanthanum, and hence the expected electronic configuration of Lanthanum(La) is [Xe] f d s4 5 61 0 2 but the actual electronic configuration of Lanthanum is
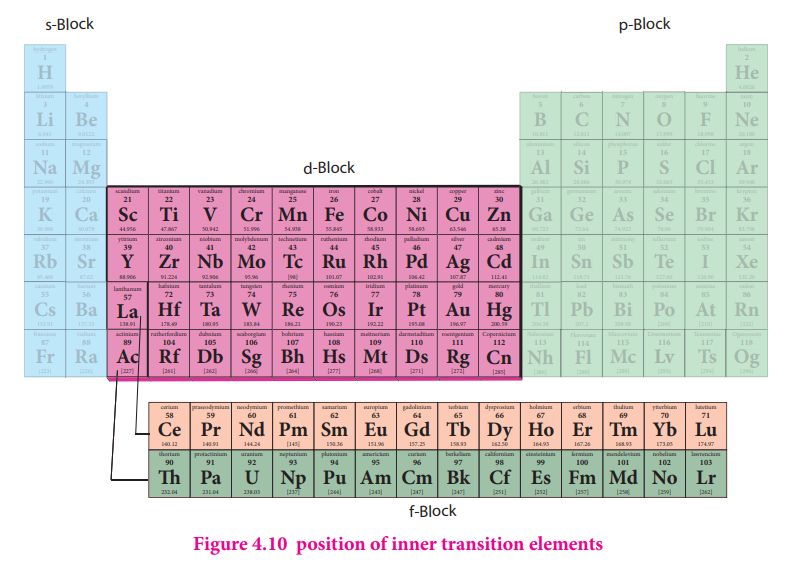
Figure 4.10 position of inner transition elements
XII U4-D-Block-Jerald Folder.indd 119 2/19/2020 4:41:37 PM
| LN6.94sod1ium22.99pota1ssium39.09rubidium | BMC d-Block9.012 10.81 12.011 14.00magnesi1 u aluminiu silico phosphorus24.30calci2 um scandium titanium vanadium chromium manganese iron cobalt nickel copper zinc40.07strontium | 915.99 18.99sulfu chlorin | N120.18argo | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A126.98gallium | S14 128.08 30.97germanium arsenic | 132.06selenium | Cl135.45bromin | Ar139.94krypto | |||||||||||
| Sc Ti V Cr Mn Fe Co Ni Cu ZnY Zr Nb Mo Tc Ru Rh Pd Ag CdLa21 Hf22 Ta23 W24 Re25 Os26 Ir27 Pt28 Au29 Hg3044.956 47.867 50.942 51.996 54.938 55.845 58.933 58.693 63.546 65.38Acyttr39ium zirRfco40nium Dbnio41bium molySg42bdenum teBhchne43tium ruHstheni44 um Mtrho45dium palladiDs46um Rgsilver47 Cncadmi48um88.906 91.224 92.906 95.96 [98] 101.07 102.91 106.42 107.87 112.41lant57hanum hafn 72ium tant 73alum tungsten74 rhenium75 osmi76um iridi77um plat 78inum gold79 merc80ury138.91 178.49 180.95 183.84 186.21 190.23 192.22 195.08 196.97 200.59actinium89 ruther 104fordium dubn105ium seab 106orgium bo107hrium hassium108 meitnerium109 darm 110stadtium roen 111tgenium Copernici112 um[227] [261] [262] [266] [264] [277] [268] [271] [272] [285] | G369.72indium | G As S Br3 3 34 372.6 74.92 78.9 79.90ti antimony tellurium iodin | Kr383.79xeno | ||||||||||||
| Rb385.46caesium | Sr387.6bariu | In49114.8thallium | Sn5118.71lead | S5121.7bismut | T5127.6poloniu | 5126.9astatine | X54131.2rado | ||||||||
| C5132.9francium | B5137.3radium | T81204.3Nahonium | P B Po A82 8 84 8207. 208.9 [209 [210Flerovium Mascovium Livermorium Tennessine | Rn8[222Oganesso | |||||||||||
| F8[223 | R8[226 | ||||||||||||||
| N113[286 | F114[289 | Mc11[289 | L11[293 | Ts11[294 | Og11[294 |
[Xe] f d s4 5 60 1 2 and it belongs to d block. Filling of 4f orbital starts from Cerium (Ce) and its electronic configuration is [Xe] f d s4 5 61 1 2 . As we move from Cerium to other elements the additional electrons are progressively filled in 4f orbitals as shown in the table.
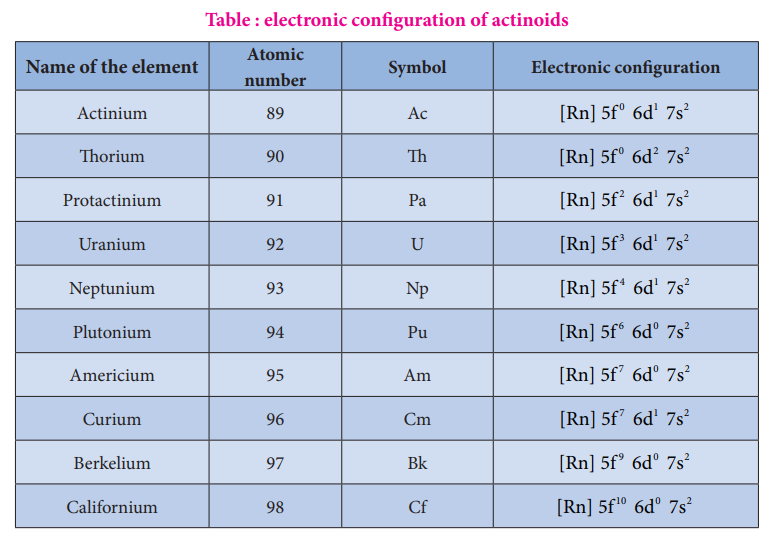
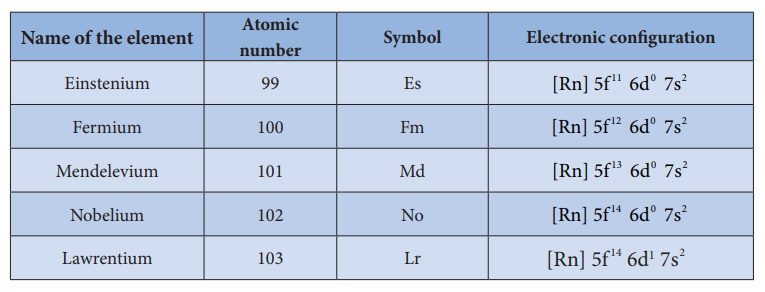
Table : electronic configuration of Lanthanum and Lanthanoids
Name of the element Atomic number Symbol Electronic configuration
Lanthanum 57 La [Xe] f d s4 5 60 1 2
Cerium 58 Ce [Xe] f d s4 5 61 1 2
Praseodymium 59 Pr [Xe] f d s4 5 63 0 2
Neodymium 60 Nd [Xe] f d s4 5 64 0 2
Promethium 61 Pm [Xe] f d s4 5 65 0 2
Samarium 62 Sm [Xe] f d s4 5 66 0 2
Europium 63 Eu [Xe] f d s4 5 67 0 2
Gadolinium 64 Gd [Xe] f d s4 5 67 1 2
Terbium 65 Tb [Xe] f d s4 5 69 0 2
Dysprosium 66 Dy [Xe] f d s4 5 610 0 2
Holmium 67 Ho [Xe] f d s4 5 611 0 2
Erbium 68 Er [Xe] f d s4 5 612 0 2
Thulium 69 Tm [Xe] f d s4 5 613 0 2
Ytterbium 70 Yb [Xe] f d s4 5 614 0 2
Lutetium 71 Lu [Xe] f d s4 5 614 1 2
In Gadolinium (Gd) and Lutetium (Lu) the 4f orbitals, are half-filled and completely filled, and one electron enters 5d orbitals. Hence the general electronic configuration of 4f series of elements can be written as [Xe] f d s4 5 61 14 0 1 2− −
Oxidation state of lanthanoids:
The common oxidation state of lanthanoids is +3. In addition to that some of the lanthanoids also show either +2 or +4 oxidation states.
Gd3+ and Lu3+ ions have extra stability, it is due to the fact that they have exactly half filled and completely filled f-orbitals respectively.their electronic c onfigurations are
[ ] [ ]
3 7
3 14
Gd : 4 Lu : 4
Xe f Xe f
XII U4-D-Block-Jerald Folder.indd 120 2/19/2020 4:41:53 PM
| Name of the element | Atomic number | Symbol | Electronic conguration |
|---|---|---|---|
| Lanthanum | 57 | La | [Xe] f45 d s601 2 |
| Cerium | 58 | Ce | [Xe] f45 d s611 2 |
| Praseodymium | 59 | Pr | [Xe] f45 d s630 2 |
| Neodymium | 60 | Nd | [Xe] f45 d s640 2 |
| Promethium | 61 | Pm | [Xe] f45 d s650 2 |
| Samarium | 62 | Sm | [Xe] f45 d s660 2 |
| Europium | 63 | Eu | [Xe] f45 d s670 2 |
| Gadolinium | 64 | Gd | [Xe] f45 d s671 2 |
| Terbium | 65 | Tb | [Xe] f45 d s690 2 |
| Dysprosium | 66 | Dy | [Xe] f45 d s610 02 |
| Holmium | 67 | Ho | [Xe] f45 d s611 02 |
| Erbium | 68 | Er | [Xe] f45 d s612 02 |
| ulium | 69 | Tm | [Xe] f45 d s613 02 |
| Ytterbium | 70 | Yb | [Xe] f45 d s614 02 |
| Lutetium | 71 | Lu | [Xe] f45 d s614 12 |
Similarly Cerium and terbium attain 4f0 and 4f7 configurations respectively in the +4 oxidation states. Eu2+ and Yb2+ ions have exactly half filled and completely filled f orbitals respectively.
The stability of different oxidation states has an impact on the properties of these elements. the following table shows the different oxidation states of lanthanoids.
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
+2 +2 +2 +2 +2
+3 +3 +3 +3 +3 +3 +3 +3 +3 +3 +3 +3 +3 +3
+4 +4 +4 +4 +4
Atomic and ionic radii:
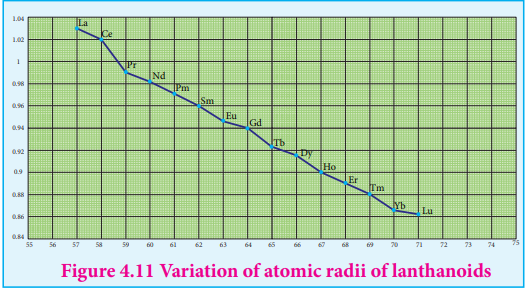
As we move across 4f series, the atomic and ionic radii of lanthanoids show gradual decrease with increse in atomic number. This decrese in ionic size is called lanthanoid contraction.
Cause of lanthanoid contraction:
As we move from one element to another in 4f series ( Ce to Lu) the nuclear charge increases by one unit and an additional electron is added into the same inner 4f sub shell. We know that 4f sub shell have a diffused shapes and therefore the shielding effect of 4f elelctrons relatively poor.hence, with increase of nuclear charge, the valence shell is pulled slightly towards nucleus. As a result, the effetive nuclear charge experienced by the 4f elelctorns increases and the size of Ln3+ ions decreases. Lanthanoid contraction of various lanthanoids is shown in the graph
Consequences of lanthanoid contraction: 1. Basicity differences
As we from Ce3+ to Lu3+ , the basic character of Ln3+ ions decrease. Due to the decrease in the size of Ln3+ ions, the ionic character of Ln OH− bond decreases (covalent character increases) which results in the decrease in the basicity. 2. Similarities among lanthanoids:
In the complete f - series only 10 pm decrease in atomic radii and 20 pm decrease in ionic radii is observed. because of this very small change in radii of lanthanoids, their chemical properties are quite similar.
0.84
0.86
0.88
0.9
0.92
0.94
0.96
0.98
1.02
1.04
55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75
La Ce
Pr Nd
Pm Sm
Eu Gd
Tb Dy
Ho Er
Tm
Yb Lu
Figure 4.11 Variation of atomic radii of lanthanoids
XII U4-D-Block-Jerald Folder.indd 121 2/19/2020 4:41:53 PM
| Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +2 | +2 | +2 | +2 | +2 | |||||||||
| +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 |
| +4 | +4 | +4 | +4 | +4 |
| LaCePrNdPmSmEu Gd | |
|---|---|
| TbDyHoErTmYb Lu |
The elements of the second and third transition series resemble each other more closely than the elements of the first and second transition series. For example
Series Element Atomic radius
3d Series Ti 132 pm
4d Series Zr 145 pm
5d Series Hf 144 pm
Actinoids:
The fourteen elements following actinium ,i.e., from thorium (Th) to lawrentium (Lr) are called actinoids. Unlike the lanthanoids, all the actinoids are radioactive and most of them have short half lives. Only thorium and uranium(U) occur in significant amount in nature and a trace amounts of Plutonium(Pu) is also found in Uranium ores.Neptunium(Np) and successive heavier elements are produced synthetically by the artificial transformation of naturally occuring elements by nuclear reactions.
Similar to lanthanoids, they are placed at the bottom of the periodic table.
Electronic configuration:
The electronic configuration of actinoids is not definite. The general valence shell electronic configuration of 5f elements is represented as [Rn]5f 0-146d0-27s2. The following table show the electronic configuration of actinoids.


Table : electronic configuration of actinoids
Name of the element Atomic number
Symbol Electronic configuration
Actinium 89 Ac [Rn] 5f d s0 1 26 7
Thorium 90 Th [Rn] 5f d s0 2 26 7
Protactinium 91 Pa [Rn] 5f d s2 1 26 7
Uranium 92 U [Rn] 5f d s3 1 26 7
Neptunium 93 Np [Rn] 5f d s4 1 26 7
Plutonium 94 Pu [Rn] 5f d s6 0 26 7
Americium 95 Am [Rn] 5f d s7 0 26 7
Curium 96 Cm [Rn] 5f d s7 1 26 7
Berkelium 97 Bk [Rn] 5f d s9 0 26 7
Californium 98 Cf [Rn] 5f d s10 0 26 7
XII U4-D-Block-Jerald Folder.indd 122 2/19/2020 4:42:01 PM
| Series | Element | Atomic radius |
|---|---|---|
| 3d Series | Ti | 132 pm |
| 4d Series | Zr | 145 pm |
| 5d Series | Hf | 144 pm |
| Name of the element | Atomic number | Symbol | Electronic conguration |
|---|---|---|---|
| Actinium | 89 | Ac | [Rn] 5f 67d s01 2 |
| orium | 90 | | [Rn] 5f 67d s02 2 |
| Protactinium | 91 | Pa | [Rn] 5f 67d s21 2 |
| Uranium | 92 | U | [Rn] 5f 67d s31 2 |
| Neptunium | 93 | Np | [Rn] 5f 67d s41 2 |
| Plutonium | 94 | Pu | [Rn] 5f 67d s60 2 |
| Americium | 95 | Am | [Rn] 5f 67d s70 2 |
| Curium | 96 | Cm | [Rn] 5f 67d s71 2 |
| B erkelium | 97 | Bk | [Rn] 5f 67d s90 2 |
| Californium | 98 | Cf | [Rn] 5f 67d s10 02 |
Name of the element Atomic number
Symbol Electronic configuration
Einstenium 99 Es [Rn] 5f d s11 0 26 7
Fermium 100 Fm [Rn] 5f d s12 0 26 7
Mendelevium 101 Md [Rn] 5f d s13 0 26 7
Nobelium 102 No [Rn] 5f d s14 0 26 7
Lawrentium 103 Lr [Rn] 5f s14 276d1
Oxidation state of actinoids:
Like lanthanoids, the most common state of actinoids is +3. In addition to that actinoids show variable oxidation states such as +2 , +3 , +4 ,+5,+6 and +7.
The elements Americium(Am) and Thorium (Th) show +2 oxidation state in some compounds , for example thorium iodide (ThI2). The elements Th , Pa, U ,Np , Pu and Am show +5 oxidation states. Np and Pu exhibit +7 oxidation state.
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr +2 +2 +3 +3 +3 +3 +3 +3 +3 +3 +3 +3 +3 +3 +3 +3 +4 +4 +4 +4 +4 +4 +4 +4 +5 +5 +5 +5 +5 +5
+6 +6 +6 +6 +7 +7 +7
Differences between lanthanoids and actinoids:
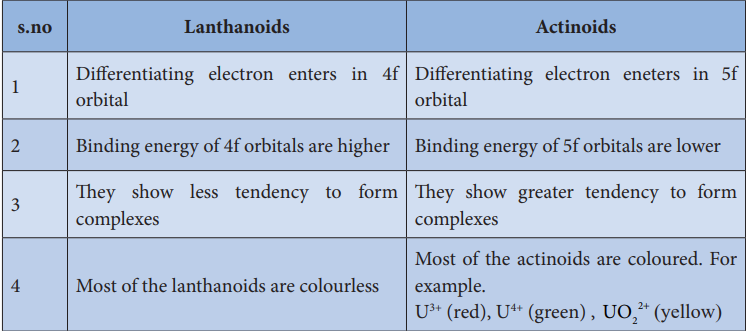
s.no Lanthanoids Actinoids
1 Differentiating electron enters in 4f orbital
Differentiating electron eneters in 5f orbital
2 Binding energy of 4f orbitals are higher Binding energy of 5f orbitals are lower
3 They show less tendency to form complexes
They show greater tendency to form complexes
4 Most of the lanthanoids are colourless Most of the actinoids are coloured. For example. U3+ (red), U4+ (green) , UO2
2+ (yellow)
XII U4-D-Block-Jerald Folder.indd 123 2/19/2020 4:42:06 PM
| Name of the element | Atomic number | Symbol | Electronic conguration |
|---|---|---|---|
| Einstenium | 99 | Es | [Rn] 5f 67d s11 02 |
| Fermium | 100 | Fm | [Rn] 5f 67d s12 02 |
| Mendelevium | 101 | Md | [Rn] 5f 67d s13 02 |
| Nobelium | 102 | No | [Rn] 5f 67d s14 02 |
| Lawrentium | 103 | Lr | [Rn] 5f 6d 7s114 2 |
| | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +2 | +2 | ||||||||||||
| +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 |
| +4 | +4 | +4 | +4 | +4 | +4 | +4 | +4 | ||||||
| +5 | +5 | +5 | +5 | +5 | +5 | ||||||||
| +6 | +6 | +6 | +6 | ||||||||||
| +7 | +7 | +7 |
| s.no | L anthanoids | Actinoids |
|---|---|---|
| 1 | Dierentiating e lectron en ters in 4f orbital | Dierentiating e lectron en eters in 5f orbital |
| 2 | Binding energ y of 4f orbitals are higher | Binding energ y of 5f orbitals are lower |
| 3 | ey s how les s t endency t o f orm complexes | ey s how g reater t endency t o f orm complexes |
| 4 | Most of the lanthanoids are colourless | Most o f t he ac tinoids a re co loured. F or example. U (red), U (green) , UO (yellow)3+ 4+ 2+2 |
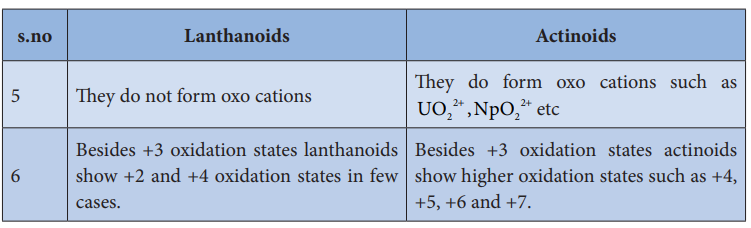
s.no Lanthanoids Actinoids
5 They do not form oxo cations They do form oxo cations such as UO NpO2
2 2
2+ +, etc
6 Besides +3 oxidation states lanthanoids show +2 and +4 oxidation states in few cases.
Besides +3 oxidation states actinoids show higher oxidation states such as +4, +5, +6 and +7.
Summary
„ IUPAC defines transition metal as an element whose atom has an incomplete d sub shell or which can give rise to cations with an incomplete d sub shell. They occupy the central position of the periodic table, between s and p block elements,
„ d- Block elements composed of 3d series (4th period) Scandium to Zinc ( 10 elements), 4d series ( 5th period) Yttrium to Cadmium ( 10 elements) and 5d series ( 6th period) Lanthanum, Haffinium to mercury.
„ the general electronic configuration of d- block elements can be written as [ ]Noble gas n d ns−( ) − −1 1 10 1 2, Here, n = 4 to 7 . In periods 6 and 7, the configuration includes (_n f_−( )2 orbital ; [ ]Noble gas n 2 f n d ns14−( ) −( ) − −1 1 10 1 2 .
„ All the transition elements are metals. Similar to all metals the transition metals are good conductors of heat and electricity. Unlike the metals of Group-1 and group-2, all the transition metals except group 11 elements are hard.
„ As we move from left to right along the transition metal series, melting point first increases as the number of unpaired d electrons available for metallic bonding increases, reach a maximum value and then decreases, as the d electrons pair up and become less available for bonding.
„ Ionization energy of transition element is intermediate between those of s and p block elements. As we move from left to right in a transition metal series, the ionization enthalpy increases as expected.
„ The first transition metal Scandium exhibits only +3 oxidation state, but all other transition elements exhibit variable oxidation states by loosing electrons from (n-1)d orbital and ns orbital as the energy difference between them is very small.
„ In 3d series as we move from Ti to Zn, the standard reduction potential E M
M
0 2+
value is approaching towards less negative value and copper has a positive
reduction potential. i.e., elemental copper is more stable than Cu2+.
XII U4-D-Block-Jerald Folder.indd 124 2/19/2020 4:42:07 PM
| s.no | Lanthanoids | Actinoids |
|---|---|---|
| 5 | ey do not form oxo cations | ey do f orm o xo c ations s uch a s UO , NpO etc2++ 22 2 |
| 6 | B esides +3 o xidation states lanthanoids show +2 a nd +4 o xidation s tates in f ew cases. | B esides +3 o xidation s tates ac tinoids show hig her oxidation states such as +4, +5, +6 and +7. |
„ Most of the compounds of transition elements are paramagnetic. Magnetic properties are related to the electronic configuration of atoms.
„ Many industrial processes use transition metals or their compounds as catalysts. Transition metal has energetically available d orbitals that can accept electrons from reactant molecule or metal can form bond with reactant molecule using its d electrons.
„ Transition metals form a number of interstitial compounds such as TiC, ZrH , Mn N etc1.92 4 .
„ Transition elements have a tendency to form coordination compounds with a species that has an ability to donate an electron pair to form a coordinate covalent bond.
„ In the inner transition elements there are two series of elements. 1) Lanthanoids ( previously called lanthanides) 2) Actinoids ( previously called actinides)
„ Lanthanoids have general electronic configuration [Xe] f d s4 5 61 14 0 1 2− −
„ The common oxidation state of lanthanoides is +3
„ As we move across 4f series, the atomic and ionic radii of lanthanoids show gradual decrease with increse in atomic number. This decrese in ionic size is called lanthanoid contraction.
„ The electronic configuration of actinoids is not definite. The general valence shell electronic configuration of 5f elements is represented as [Rn]5f 0-146d0-27s2.
„ Like lanthanoids, the most common state of actinoids is +3. In addition to that actinoids show variable oxidation states such as +2 , +3 , +4 ,+5,+6 and +7.
EVALUATION
Choose the best answer:
1. Sc( Z=21) is a transition element but Zinc (z=30) is not because
a) both Sc3+ and Zn2+ ions are colourless and form white compounds.
b) in case of Sc, 3d orbital are partially filled but in Zn these are completely filled
c) last electron as assumed to be added to 4s level in case of zinc
d) both Sc and Zn do not exhibit variable oxidation states
2. Which of the following d block element has half filled penultimate d sub shell as well as half filled valence sub shell?
a) Cr b) Pd
c) Pt d) none of these
XII U4-D-Block-Jerald Folder.indd 125 2/19/2020 4:42:08 PM
3. Among the transition metals of 3d series, the one that has highest negative M M
2+( ) standard electrode potential is
a) Ti b) Cu c) Mn d) Zn
4. Which one of the following ions has the same number of unpaired electrons as present in V3+?
a) Ti3+ b) Fe3+
c) Ni2+ d) Cr3+
5. The magnetic moment of Mn2+ ion is
a) 5.92BM b) 2.80BM
c) 8.95BM d) 3.90BM
6. the catalytic behaviour of transition metals and their compounds is ascribed mainly due to
a) their magnetic behaviour
b) their unfilled d orbitals
c) their ability to adopt variable oxidation states
d) their chemical reactivity
7. The correct order of increasing oxidizing power in the series
a) VO Cr O < MnO2 +
2< − − 7
2 4 b) Cr O < VO MnO2 2
+ 7
2 4
− −<
c) Cr O MnO < VO2 2 +
7 2
4 − −< d) MnO < Cr O VO2 2
+ 4 7
2− − <
8. In acid medium, potassium permanganate oxidizes oxalic acid to
a) oxalate b) Carbon dioxide
c) acetate d) acetic acid
9. Which of the following statements is not true?
a) on passing H2S, through acidified K2Cr2O7 solution, a milky colour is observed.
b) Na2Cr2O7 is preferred over K2Cr2O7 in volumetric analysis
c) K2Cr2O7 solution in acidic medium is orange in colour
d) K2Cr2O7 solution becomes yellow on increasing the PH beyond 7
10. Permanganate ion changes to ________ in acidic medium
a) MnO 4 2− b) Mn 2+
c) Mn 3+ d) MnO 2
XII U4-D-Block-Jerald Folder.indd 126 2/19/2020 4:42:15 PM
11. How many moles of I2 are liberated when 1 mole of potassium dichromate react with potassium iodide?
a) 1 b) 2
c) 3 d) 4
12. The number of moles of acidified KMnO4 required to oxidize 1 mole of ferrous oxalate(FeC2O4) is
a) 5 b) 3 c) 0.6 d) 1.5
13. Which one of the following statements related to lanthanons is incorrect?
a) Europium shows +2 oxidation state.
b) The basicity decreases as the ionic radius decreases from Pr to Lu.
c) All the lanthanons are much more reactive than aluminium.
d) Ce4+ solutions are widely used as oxidising agents in volumetric analysis.
14. Which of the following lanthanoid ions is diamagnetic?
a) Eu2+ b) Yb2+
c) Ce2+ d) Sm2+
15. Which of the following oxidation states is most common among the lanthanoids?
a) 4 b) 2
c) 5 d) 3
16. Assertion : Ce4+ is used as an oxidizing agent in volumetric analysis. Reason: Ce4+ has the tendency of attaining +3 oxidation state. a) Both assertion and reason are true and reason is the correct explanation of assertion. b) Both assertion and reason are true but reason is not the correct explanation of assertion. c) Assertion is true but reason is false. d) Both assertion and reason are false.
17. The most common oxidation state of actinoids is
a) +2 b) +3
c) +4 d) +6
18. The actinoid elements which show the highest oxidation state of +7 are
a) Np, Pu ,Am b) U, Fm, Th
c) U, Th, Md d) Es, No, Lr
XII U4-D-Block-Jerald Folder.indd 127 2/19/2020 4:42:15 PM
19. Which one of the following is not correct?
a) La(OH)3 is less basic than Lu(OH)3
b) In lanthanoid series ionic radius of Ln3+ ions decreases
c) La is actually an element of transition metal series rather than lanthanide series
d) Atomic radii of Zr and Hf are same because of lanthanide contraction
Answer the following questions:
1. What are transition metals? Give four examples.
2. Explain the oxidation states of 4d series elements.
3. What are inner transition elements?
4. Justify the position of lanthanides and actinides in the periodic table.
5. What are actinides? Give three examples.
6. Describe the preparation of potassium dichromate.
7. What is lanthanide contraction and what are the effects of lanthanide contraction?
8. complete the following
a. MnO H b. C H CH
c. MnO
6 5 3
− ++ → →
? ?acidified
KMnO
4 Red hot
2 7
Fe d. KMnO e. Cr O I H
− +
∆
− − +
+ → →
+ + →
? ?
? ?f. Na Cr O KCl2 2 7
+ →
9. What are interstitial compounds?
10. Calculate the number of unpaired electrons in Ti3+ , Mn2+ and calculate the spin only
magnetic moment.
11. Write the electronic configuration of Ce4+ and Co2+.
12. Explain briefly how +2 states becomes more and more stable in the first half of the first row
transition elements with increasing atomic number.
13. Which is more stable? Fe3+ or Fe2+ - explain.
14. Explain the variation in E0 M3+ M2+/
3d series.
XII U4-D-Block-Jerald Folder.indd 128 2/19/2020 4:42:16 PM
15. Compare lanthanides and actinides.
16. Explain why Cr2+ is strongly reducing while Mn3+ is strongly oxidizing.
17. Compare the ionization enthalpies of first series of the transition elements.
18. Actinoid contraction is greater from element to element than the lanthanoid contraction,
why?
19. Out of Lu(OH)3 and La(OH)3 which is more basic and why?
20. Why europium (II) is more stable than Cerium (II)?
21. Why do zirconium and Hafnium exhibit similar properties?
22. Which is stronger reducing agent Cr2+ or Fe2+?
23. The E0 M2+ M/
value for copper is positive. Suggest a possible reason for this.
24. Describe the variable oxidation state of 3d series elements.
25. Which metal in the 3d series exhibits +1 oxidation state most frequently and why?
26. Why first ionization enthalpy of chromium is lower than that of zinc?
27. Transition metals show high melting points why?
XII U4-D-Block-Jerald Folder.indd 129 2/19/2020 4:42:17 PM