Classification of solids:
We can classify solids into the following two major types based on the arrangement of their constituents.
(i) Crystalline solids
(ii) Amorphous solids.
The term crystal comes from the Greek word “krystallos” which means clear ice. This term was first applied to the transparent quartz stones, and then the name is used for solids bounded by many flat, symmetrically arranged faces.

A crystalline solid is one in which its constituents (atoms, ions or molecules), have an orderly arrangement extending over a long range. The arrangement of such constituents in a crystalline solid is such that the potential energy of the system is at minimum. In contrast, in amorphous solids (In Greek, amorphous means no form) the constituents are randomly arranged. The following table shows the differences between crystalline and amorphous solids.
Table 6.1 differences between crystalline and amorphous solids
| S.no | Cr ystalline solids | Amorphous solids |
|---|---|---|
| 1 | Long range orderly arrangement of constituents. | Short range, random arrangement of constituents. |
| 2 | De:railway_car:nite shape | Irregular shape |
| 3 | Generally cr ystalline solids are anisotropic in nature | :car:ey are isotropic* like liquids |
| 4 | :car:ey are true solids | :car:ey are considered as pseudo solids (or) super cooled liquids |
| 5 | De:railway_car:nite Heat of fusion | Heat of fusion is not de:railway_car:nite |
| 6 | :car:ey have sharp melting points. | Gradually so:bullettrain_front:en over a range of temperature and so can be moulded. |
| 7 | Examples: NaCl , diamond etc., | Examples: Rubber , plastics, glass etc |
Table 6.1 differences between crystalline and amorphous solids
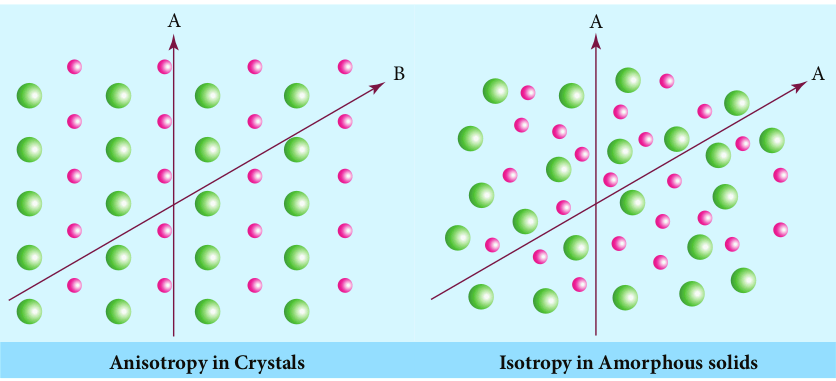
*Isotropy
Isotropy means uniformity in all directions. In solid state isotropy means having identical values of physical properties such as refractive index, electrical conductance etc., in all directions, whereas anisotropy is the property which depends on the direction of measurement. Crystalline solids are anisotropic and they show different values of physical properties when measured along different directions. The following figure illustrates the anisotropy in crystals due to different arrangement of their constituents along different directions.