NITRO COMPOUNDS
Nitro compounds are considered as the derivaties of hydrocarbons. If one of the hydrogen atom of hydrocarbon is replaced by the -NO2 group, the resultant organic compound is called a nitrocompound.
Classification of nitrocompounds
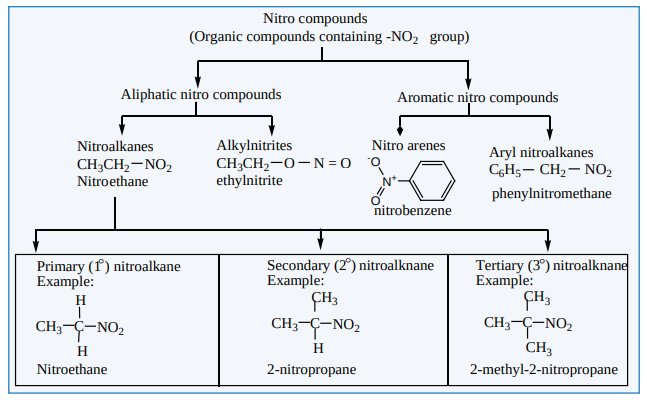
Nitroalkanes are represented by the formula, R-NO2 where R is an alkyl group (CH-)n 2n+1 . Nitroalkanes are further classified into primary, secondary, tertiary nitroalkanes on the basis of type of carbon atom to which the nitro (-NO )2 group is attached.
Nomenclature of nitroalkanes
In the IUPAC nomenclature, the nitroalkanes are named by adding prefix nitro before the name of alkane, the position of the nitro group is indicated by number.
| Compound (common name, Structural formula, IUPAC Name) |

|
|---|---|
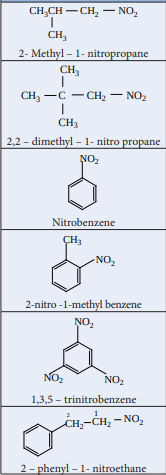
|
|
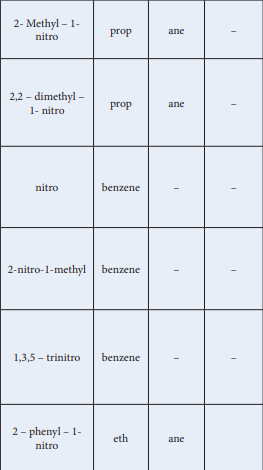
| Compound(common name, Structural formula, IUPAC Name) | IUPAC Name | |||
|---|---|---|---|---|
| Prefix with position number | Root used | Primary suffx | Secondary Suffix | |
| \ch{H3C-CH3} CH CH CH NO2- Met3CHhyl – 1- ni 2tropropa 2ne3 | 2- Methyl – 1- nitro | prop | ane | – |
| CHCH C CH NO3CH3 22,2 – dimethyl – 1- nitro propane3 | 2,2 – dimethyl – 1- nitro | prop | ane | – |
| NO2Nitrobenzene | nitro | benzene | – | – |
| CH3 NO2-nitro -1-methyl benzen2 e | 2-nitro-1-methyl | benzene | – | – |
| NO21,3,5 – tNO rinitrobenzen NO e2 2 | 1,3,5 – trinitro | benzene | – | – |
| CH CH NO12 – phenyl – 1- ni2 2 tr 2oethan 2e | 2 – phenyl – 1- nitro | eth | ane |
ISOMERISM
Nitroalkanes exhibit chain and position isomerism among their own class and functional isomerism with alkyl nitrites and special type tautomerism can also exist in nitro alkanes having an α-H atom. For example, nitro compounds having the molecular formula C4H9NO2 exhibit the following isomerisms. Isomerism Structural formula of isomers Chain isomerism: They differ in the length of carbon chain.
| Isomerism | Structural formula of isomers |
|---|---|
| Chain isomerism: They differ in the length of carbon chain. | CH3CH2CH2CH2NO21 - nitrobutane and CH3CHCH2 NO2 CH3 2 - methyl - 1-nitropropane |
| Positionisomerism: ey dier in the position of nitro group. | CH3CH2CH2CH2 NO2,1 - nitrobutane CH3CHCH2CH3 NO2 and CH3 C CH3 CH3 NO2 2 - nitrobutane 2 - methyl - 2-nitropropane |
| Functional isomerism:Nitroalkanesexhibit functional isomerism with alkylnitrites | CH3CH2CH2CH2 NO2 1 - nitrobutane and CH3CH2CH2CH2 O N = O butyl nitrite |
Tautomerism: Primary and secondary nitroalkanes, having α-H , also show an equilibrium mixture of two tautomers namely nitro – and aci – form
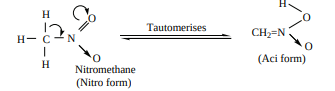
Tertiary nitro alkanes donot exhibit tautomerism due to absence of α-H atom.
| S.No. | Nitro form | Aci – form |
|---|---|---|
| 1. | Less acidic | More acidic |
| 2. | Dissolves in NaOH slowly | Dissolves in NaOH instantly |
| 3. | Decolourises FeCl solution3 | With gives reddish brown colourFeCl3 |
| 4. | Electrical conductivity is low | Electrical conductivity is high |
Acidic nature of nitro alkanes
The α-H atom of 1 & 2 nitroalkanes show acidic character because of the electron with drawing effect of NO2 group. These are more acidic than aldehydes, ketones, ester and cyanides. Nitroalkanes dissolve in NaOH solution to form a salt. Aci – nitro derivatives are more acidic than nitro form. When the number of alkyl group attached to α carbon increases, acidity decreases. due to +I effect of alkyl groups.
CH3NO3 > CH3CH2 NO2 NO2> CH3 CH3 CH NO2
Preparation of nitroalkanes
- From alkyl halides: (Laboratory method)
a) Alkyl bromides (or) iodides on heating with ethanolic solution of potassium nitrite gives nitroethane.
\[\underset{\text{Ethyl bromide}}{CH_{3}CH_{2}Br}+KNO_{2} \xrightarrow{Ethanol} \underset{\text{Nitroethane}}{CH_{3}CH_{2}NO_{2}+KBr} \]The reaction follows SN2 mechanism.
This method is not suitable for preparing nitrobenzene because the bromine directly attached to the benzene ring cannot be cleaved easily.
2) Vapour phase nitration of alkanes: (Industrial method)
Gaseous mixture of methane and nitric acid passed through a red hot metal tube to give nitromethane.

Except methane, other alkanes (upto n – hexane) give a mixture of nitroalkanes due to C-C cleavage. The individual nitro alkanes can be separated by fractional distillation.
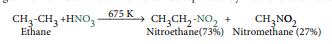
3) From a- halocarboxylic acid
α-choloroacetic acid when boiled with aqueous solution of sodium nitrite gives nitromethane.
4) Oxidation of tert – alkyl amines tert – butyl amine is oxidised with aqueous KMnO4 to give tert – nitro alkanes.
5) Oxidation of Oximes Oxidation of acetaldoxime and acetoneoxime with trifluoroperoxy acetic acid gives
nitroethane (10) and 2 – nitropropane (20) respectively.
CH3CHNOH -> CH3CH2NO2
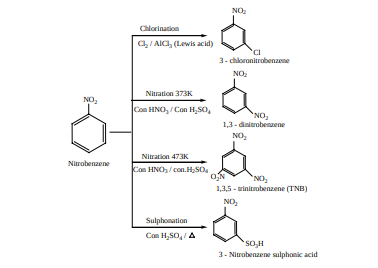
Preparation of Nitroarenes 1) By Direct nitration
When benzene is heated at 330K with a nitrating mixture (Con.HNO + Con.H SO3 2 4 ) , electrophilic substitution takes place to form nitro benzene. (Oil of mirbane)
On direct nitration of nitrobenzene m- dinitrobenzene is obtained
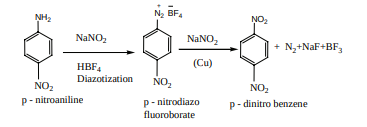
2) Indirect method Nitration of nitro benzene gives m-dinitrobenzene. The following method is adopted for the preparation of p-dinitrobenzene. For example
Physical properties of nitro alkane
The lower nitroalkanes are colourless pleasant smelling liquids, sparingly soluble in water,
but readily soluble in organic solvents like benzene, acetone etc… They have high boiling points because of their highly polar nature. Alkylnitrites have lower boiling points than nitro alkanes. ### Chemical properties of nitroalkanes
Nitroalkanes undergo the following common reactions. i. Reduction ii. Hydrolysis iii. Halogenations
i. Reduction of nitroalkanes Reduction of nitroalkanes has important synthetic applications. The various reduction stages of nitro group are given below.
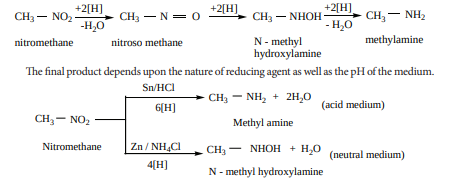
Reduction of alkyl nitrites
Ethylnitrite on reduction with Sn / HCl gives ethanol
CH3CHNOH -> CH3CH2NO2
ii. Hydrolysis of nitroalkanes
Hydrolysis can be effected using conc. HCl or conc. H SO2 4 . Primary nitroalkanes on hydrolysis gives carboxylic acid, and the secondary nitroalkanes give ketones. The tertiary nitroalkanes have no reaction.
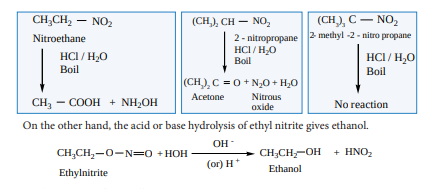
iii. Halogenation of nitroalkanes Primary and secondary nitroalkanes on treatement with Cl or Br2 2 in the presence of
NaOH give halonitroalkanes. The α - H atom of nitroalkanes are successively replaced by halogen atoms.
CH3 NO2 +3Cl2 NaOH CCl3 NO2 + 3HCl
Chloropicrin (trichloronitromethane)
Toxicity Nitroethane is suspected to cause genetic damage and be harmful to the nervous system.
Chemical Properties of nitrobenzene
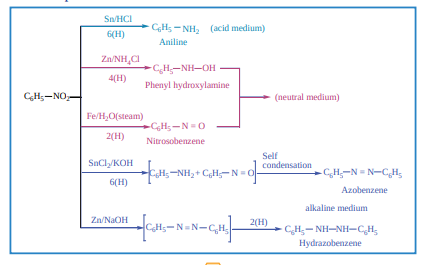
Electrolytic reduction:
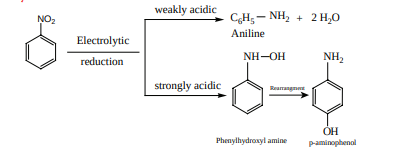
Reduction of catalytic and metal hydrides Nitrobenzene reduction with Ni (or) Pt, (or) LiAlH4 to give aniline
C6H5 - NO2 + 6 [H] Ni (or) Pt / H2 (or) LiAH4 C6H5 - NH2 + 2 H2O Selective reduction of polynitro compounds NO2 NO2 + 3 (NH4)2 S NO2 NH2 + 6NH3 + 2H2O + 3S m-dintrobenzene m-nitroaniline
Electrophilic substitution reaction The electrophilic substitution reactions of nitrobenzene are usually very slow and vigorous reaction condition have to be employed (- NO2 group is stongly deactivating and m – directing).