Thermodynamics of cell reactions
We have just learnt that in a galvanic cell, the chemical energy is converted into electrical energy. The electrical energy produced by the cell is equal to the product of the total charge of electrons and the emf of the cell which drives these electrons between the electrodes.
If ‘n’ is the number of moles of electrons exchanged between the oxidising and reducing agent in the overall cell reaction, then the electrical energy produced by the cell is given as below.
Electrical energy = Charge of ’n’ mole of electrons Ecel× l ……(9.20) Charge of 1 mole of electrons = one Faraday (1F)
Charge of ’n’ mole ∴ of electrons = nF Equation …….(9.20) Electrical energy = nF⇒ E cell ……(9.21)
Charge of one elctron = 1.602 × 10^-19
∴charge one mole of elctron = 6.023×10^23×1.602×10^-49C
=96488C
i.e., 1F 96500 C
This energy is used to do the electric work. Therefore the maximum work that can be obtained from a galvanic cell is
(Wmax)cell=-nFEcell …..(9.22)
Here the (-) sign is introduced to indicate that the work is done by the system on the surroundings.
We know from the Second Law of thermodynamics that the maximum work done by the system is equal to the change in the Gibbs free energy of the system.
i.e., Wmax = ∆G …..(9.23) From (9.22) and (9.23),
∆G = - nFE cell .....(9.24)
For a spontaneous cell reaction, the DG should be negative. The above expression (9.24)indicates that Ecell should be positive to get a negative DG value. When all the cell components are in their standard state, the equation (9.24) becomes
∆Go=- nFEo cell ……..(9.25)
We know that the standard free energy change is related to the equilibrium constant as per the following expression.
∆Go= -RT lnKeq …..(9.26)
Comparing (9.25) and (9.26), nFEocell= RT lnK eq
⇒ Eocell = 2.303 RT/ nF log K eq …..(9.27)
Nernst equation
Nernst equation is the one which relates the cell potential and the concentration of the species involved in an electrochemical reaction. Let us consider an electrochemical cell for which the overall redox reaction is,
xA + yB = lC+mD
The reaction quotient Q for the above reaction is given below
Q = [C]l [D]m/[A]x [B]y
…..(9.28) We have already learnt that,
∆G = ∆Go+RT InQ ……..(9.29)
The Gibbs free energy can be related to the cell emf as follows [∴equation (9.24) and (9.25)]
∆G = - nFEcell ; ∆Go= - nFEocell
Substitute these values and Q from (9.28) in the equation (9.29)
(9.29) ⇒ = - nFE - nFE + RT ln [C] [D] [A] [B]cell cell
o m
y
l
x …..(9.30)
Divide the whole equation (9.30) by (-nF)
(9.25) E = E - RT nF
[C] [D] [A] [B]cell cell
m
y⇒ ln l
x
(or) E = E - 2.303RT nF
log [C] [D] [A] [B]cell cell
m
y
l
x …..(9.31)
The above equation (9.31) is called the Nernst equation
At 25 o C (298K), the above equation (9.31) becomes,
E = E - 2.303 8.314 298 n(96500)
log [C] [D] [A] [Bcell cell
o m
x
× × l
]
E = E - 0.0591 n
y
cell cell log [C] [D]
[A] [B]
R = 8.314 JK m
y
-1 l
x
∴ mol T = 298 K. 1 F = 96500 C mol
-1
-1
…..(9.32)
Let us calculate the emf of the following cell at 25 Co using Nernst equation.
Cu (s) Cu (0.25 aq, M) Fe (0.005 aq M) Fe (0.1 aq M) Pt (s)2+ 3+ 2+
Given : E = 0.77V and E = 0.34 Vo
Fe Fe
o
Cu Cu3+ 2+ 2+( ) ( ) Half reactions are Cu (s) Cu (aq) + 2e 2+ -→ ….. (1) 2 Fe (aq)+2e 2Fe (aq) 3+ - 2+→ ….. (2)
the overall reaction is
Cu (s) + 2 Fe (aq) Cu (aq) + 2 Fe (aq) 3+ 2+ 2+→ , and n = 2
Apply Nernst equation at .25 Co .
E =E log [Cu ][Fe ] [Fe ]
cell cell o
2+ 2+ 2
3+ 2 − 0 0591
2 . [Cu (s)] = 1[ ]
E = E + (Ecell o
ox Cu Cu red Fe Fe2+ 3+ 2+
( ) )
Given standard reduction potential of Cu Cu2+ is 0.34V
∴ (E ) = -0.34Vox o
Cu Cu2+
(E ) = 0.77Vred o
Fe Fe3+ 2+
∴ E = - 0.34 + 0.77 E = 0.43V
cell
cell
∴ × E = 0.43 - 0.0591 2cell log ( . )( . )
( . ) 0 25 0 1
0 005
2
2 = log (0.25)(0.1)
= log 25 10 1 10
= l
2
-2 -2
( . )0 005
25 10
2
6
× × × × −
og 10 = 2 log = 2.
2
1010 = 0.43 - 0.0591
2 = 0.43 - 0.0591 = 0.3709V.
× 2
Evaluate yourself
The electrochemical cell reaction of the Daniel cell is
Zn (s) + Cu (aq) Zn (aq)+Cu (s)2+ 2+→ What is the change in the cell voltage on increasing the ion concentration in the anode
compartment by a factor 10?
Electrolytic cell and electrolysis
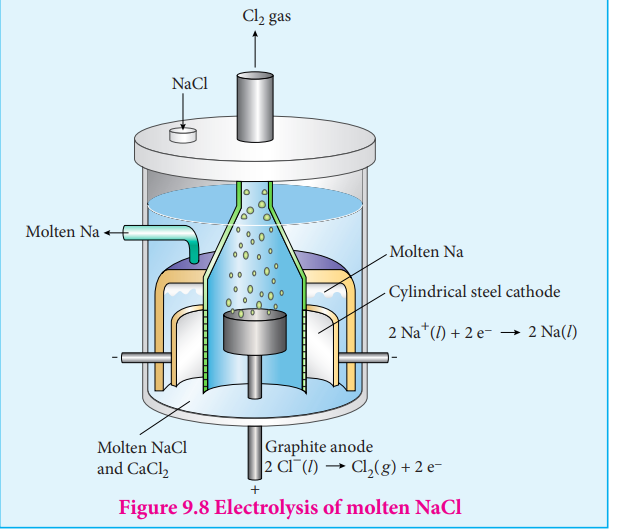
Electrolysis is a process in which the electrical energy is used to cause a non-spontaneous chemical reaction to occur; the energy is often used to decompose a compound into its elements. The device which is used to carry out the electrolysis is called the electrolytic cell. The electrochemical process occurring in the electrolytic cell and galvanic cell are the reverse of each other. Let us understand the function of a electrolytic cell by considering the electrolysis of molten sodium chloride.
The electrolytic cell consists of two iron electrodes dipped in molten sodium chloride and they are connected to an external DC power supply via a key as shown in the figure (9.8). The electrode which is attached to the negative end of the power supply is called the cathode, and the one which attached to the positive end is called the anode. Once the key is closed, the external DC power supply drives the electrons to the cathode and at the same time pull the electrons from the anode.
Cell reactions
Na+ ions are attracted towards cathode, where they combine with the electrons and reduced to liquid sodium.
Cathode (reduction)
Na ( )+e Na ( + -_l l_→ ) E - Vo = 2 71.
Similarly, Cl– ions are attracted towards anode where they lose their electrons and oxidised to chlorine gas.
Anode (oxidation)
2Cl Cl (g) + 2e - 2
-( )l → E = -1.36o V
Molten NaCl and CaCl2
Molten Na
Cl2 gas
Molten Na
Cylindrical steel cathode
Graphite anode 2 Cl−(l) Cl2( g) + 2 e−
2 Na+(l) + 2 e− 2 Na(l)
NaCl
Figure 9.8 Electrolysis of molten NaCl
|——|——|——|——|——|——|——|——|
The overall reaction is,
2Na Cl 2Na Cl + - 2( ) ( ) ( ) ( )l l l g+ → +2 E = - 4.07Vo
The negative Eo value shows that the above reaction is a non spontaneous one. Hence, we have to supply a voltage greater than 4.07V to cause the electrolysis of molten NaCl.
In electrolytic cell, oxidation occurs at the anode and reduction occur at the cathode as in a galvanic cell, but the sign of the electrodes is the reverse i.e., in the electrolytic cell cathode is –ve and anode is +ve.
Faraday’s Laws of electrolysis
First Law
The mass of the substance (m) liberated at an electrode during electrolysis is directly proportional to the quantity of charge (Q) passed through the cell.
i.e m Qα
We know that the charge is related to the current by the equation I = Q t
Q = It⇒ ∴ m It (or) m = Z It
α
….. (9.33) Where is Z is known as the electro chemical equivalent of the substance produced of the
electrode.
When, I = 1A and t = 1sec, Q = 1C , in such case the equation (9.32) becomes, (9.33)
⇒ m = Z …..(9.34)
Thus, the electrochemical equivalent is defined as the amount of substance deposited or liberated at the electrode by a charge of 1 coulomb.
Electro chemical equivalent and molar mass Consider the following general electrochemical redox reaction M (aq)+ne M(s)n+ - → We can infer from the above equation that ‘n’ moles of electrons are required to precipitate
1 mole of Mn+ as M(s). The quantity of charge required to
precipitate one mole of Mn+ = Charge of ’n’ moles of electrons
= nF In other words, the mass of substance deposited by one coulomb of charge
Electrochemical equivalent of M = Molarmass of M n (96500)
n+
(or)
Z = Equivalent mass 96500
…..(9.35)
Second Law
NiSO4 (aq)
Rh Key
CuSO4 (aq) CoSO4 (aq)
+ – + – +
+
–
– A
Figure 9.9 Electrolysis of various electrolytes using same quantity of charge
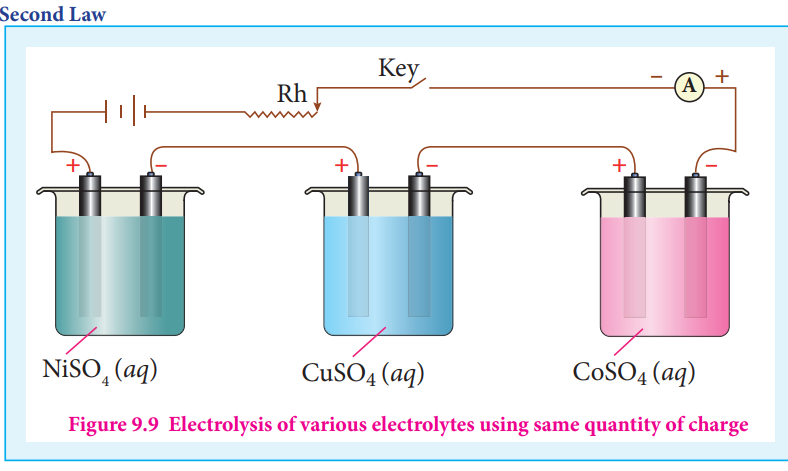
When the same quantity of charge is passed through the solutions of different electrolytes, the amount of substances liberated at the respective electrodes are directly proportional to their electrochemical equivalents.
Let us consider three electrolytic cells connected in series to the same DC electrical source as shown in the figure 9.9. Each cell is filled with a different electrolytes namely NiSO4, CuSO4 and CoSO4, respectively.
When Q coulomb charge is passed through the electrolytic cells the masses of Nickel, copper and cobalt deposited at the respective electrodes be m m and m Ni Cu Co, , respectively.
According to Faraday’s second Law,
m Z , m Z and m Z (or)
m Z
= m Z
Ni Ni Cu Cu Co Co
Ni
Ni
Cu
α α α
Cu
Co
Co
= m Z
…..(9.36) Example
A solution of silver nitrate is electrolysed for 20 minutes with a current of 2 amperes. Calculate the mass of silver deposited at the cathode.
Electrochemical reaction at cathode is Ag +e Ag (reduction)+ - →
m = ZIt Z = molarmass of Ag = 108 1 96500
m = 108 gm ( )96500 ×
ol C mol
C I = 2A
m =
-1
-196500 2400×
2.68 g. t = 20 60S = 1200 S
× It = 2A S = 2×1200 400C
Evaluate yourself A solution of a salt of metal was electrolysed for 15 minutes with a current of 0.15 amperes. The mass of the metal deposited at the cathode is 0.783g. calculate the equivalent mass of the metal.
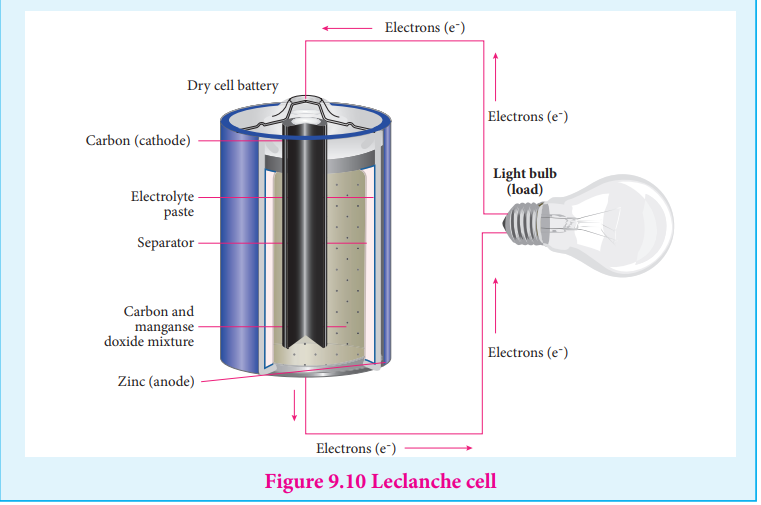
Batteries Batteries are indispensable in the modern electronic world. For example, Li – ion batteries
are used in cell phones, dry cell in flashlight etc…. These batteries are used as a source of direct current at a constant voltage. We can classify them into primary batteries (non – rechargeable) and secondary batteries (rechargeable). In this section, we will briefly discuss the electrochemistry of some batteries. Leclanche cell Anode : Zinc container Cathode : Graphite rod in contact with MnO2
Electrolyte : ammonium chloride and zinc chloride in water Emf of the cell is about 1.5V Cell reaction Oxidation at anode
Zn (s) Zn (aq)+2e 2+ -→ …..(1) Reduction at cathode
2 NH (aq) + 2e 2NH (aq) + H (g) 4 -
3 2 + → …..(2)
The hydrogen gas is oxidised to water by MnO2
H (g) + 2 MnO (s) Mn O (s) + H O ( ) 2 2 2 3 2→ l …..(3) Equation (1) + (2)+(3) gives the overall redox reaction
Dry cell battery
Carbon (cathode)
Electrolyte paste
Separator
Carbon and manganse
doxide mixture
Zinc (anode)
Electrons (e-)
Electrons (e-)
Electrons (e-)
Electrons (e-)
Light bulb (load)
Figure 9.10 Leclanche cell
| ttery | ElectrLi |
|---|
| Electr |
57
Zn (s) + 2NH (aq) + 2 MnO (s) Zn (aq) + Mn O (s) + H4 2 2+
2 3 2 + → O + NH …… (4)( )l 2 3
Ammonia produced at the cathode combines with Zn2+ to form a complex ion
Zn (NH (aq)3 )4
2[ ] + . As the reaction proceeds, the concentration of NH4 + will decrease and
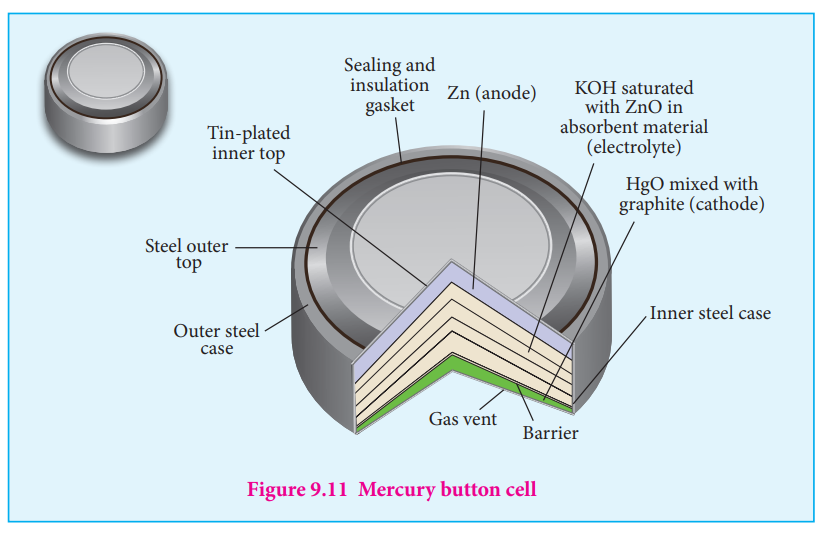
the aqueous NH3 will increase which lead to the decrease in the emf of cell. Mercury button cell
Anode : zinc amalgamated with mercury Cathode : HgO mixed with graphite Electrolyte : Paste of KOH and ZnO Oxidation occurs at anode : Zn(s) + 2OH (aq) ZnO(s) + H O 2e
0 -
from KOH
+2
2 -→ +( )l
Reduction occurs at cathode : HgO(s) + H O (l) + 2e Hg(l) + 2OH (aq) +2
2 -
0 -→
Overall reaction : Zn (s) + HgO (s) ZnO (s) + Hg → ( )l Cell emf : about 1.35V.
Uses : It has higher capacity and longer life. Used in pacemakers, electronic watches, cameras etc…
Outer steel case
Steel outer top
Tin-plated inner top
Sealing and insulation
gasket Zn (anode)
HgO mixed with graphite (cathode)
KOH saturated with ZnO in
absorbent material (electrolyte)
Inner steel case
Barrier Gas vent
Figure 9.11 Mercury button cell
We have already learnt that the electrochemical reactions which take place in a galvanic cell may be reversed by applying a potential slightly greater than the emf generated by the cell. Th is principle is used in secondary batteries to regenerate the original reactants. Let us understand the function of secondary cell by considering the lead storage battery as an example
Lead storage battery Anode : spongy lead Cathode : lead plate bearing PbO2
Electrolyte : 38% by mass of H SO2 4 with density 1.2g / mL. Oxidation occurs at the anode
Pb(s) Pb (aq)+2e2+ -→ …..(1) The Pb2+ ions combine with SO4
-2 to from PbSO4 precipitate. Pb (aq) + SO (aq) PbSO (s)2+
4 4 2− → …..(2)
Reduction occurs at the cathode PbO (s) + 4 H (aq) + 2e Pb (aq) + 2H O 2
+ - 2+ 2→ ( )l ……(3)
The Pb 2+ ions also combine with SO4
2- ions from sulphuric acid to form PbSO4 precipitate. Pb (aq) + SO (aq) PbSO
2+
4 4
2− → (s) …..(4) The Overall reactions is Equation (1) + (2) + (3) + (4)
Pb (s) + PbO (s) + 4H (aq) + 2SO (aq) 2 PbSO (s) 2
4 4
2− → + 2H O 2
( )l The emf of a single cell is about 2V . Usually six such cells are combined in series to
produce 12volt The emf of the cell depends on the concentration of H SO
2 4 . As the cell reaction uses SO4
2−
ions, the concentration H SO2 4 decreases. When the cell potential falls to about 1.8V, the cell has to be recharged. Recharge of the cell
As said earlier, a potential greater than 2V is applied across the electrodes, the cell reactions that take place during the discharge process are reversed. During recharge process, the role of anode and cathode is reversed and H SO2 4 is regenerated.
Oxidation occurs at the cathode ( now act as anode) PbSO (s) + 2H O PbO (s) + 4 H (aq) + SO (aq +2
4 2
+4
2 +
4( ) -l → 2 ) + 2e-
Reduction occurs at the anode (now act as cathode) PbSO (s) + 2e Pb(s) + SO (aq)4 4 - -→ 2
Overall reaction 2PbSO (s) + 2H O Pb (s) + PbO (s) + 4H (aq) SO
4 2 2
4
2- ( )l → + 2 (aq).
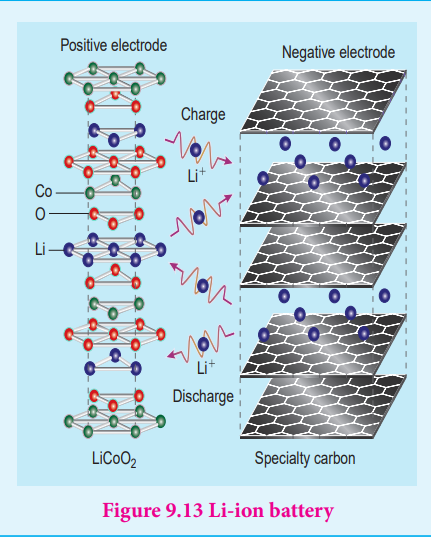
Thus, the overall cell reaction is exactly the reverse of the redox reaction which takes place while discharging . Uses: Used in automobiles, trains, inverters etc… The lithium – ion Battery Anode : Porus graphite Cathode : transition metal oxide
such as CoO2. Figure 9.12 lithium – ion Battery

Electrolyte : Lithium salt in an organic solvent
At the anode oxidation occurs
Li (s) Li (aq) + e+ -→
At the cathode reduction occurs Li + CoO (s) + e Li CoO (s) +
2 -
2→
Overall reactions Li (s) + CoO LiCoO (s)2 2→
Both electrodes allow Li+ ions to move in and out of their structures.
During discharge, the Li+ ions produced at the anode move towards cathode through the non – aqueous electrolyte. When a potential greater than the emf produced by the cell is applied across the electrode, the cell reaction is reversed and now the Li+ ions move from cathode to anode where they become embedded on the porous graphite electrode. This is known as intercalation.
Uses :
Used in cellular phones, laptops, computers, digital cameras, etc…
Fuel cell
The galvanic cell in which the energy of combustion of fuels is directly converted into electrical energy is called the fuel cell. It requires a continuous supply of reactant to keep functioning. The general representation of a fuel cell is follows
Fuel | Electrode | Electrolyte | Electrode | Oxidant
Let us understand the function of fuel cell by
Negative electrodePositive electrode
Co O
Li
Charge
Li
Li
Discharge
Specialty carbonLiCoO2
Figure 9.13 Li-ion battery
Porous gas diffusion layer
Anode
e e
H2 2H 2e 1/2O2 2H 2e H2O
H2 O2
Cathode
H2O
H
|——|
|——|
|——|——|——|——|——|——|——| | Anode |
| e |
| H |
|---|
| e |Cathode |
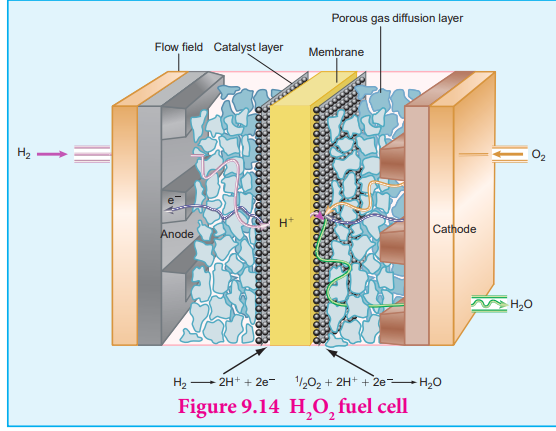
considering hydrogen – oxygen fuel cell. In this case, hydrogen act as a fuel and oxygen as an oxidant and the electrolyte is aqueous KOH maintained at 200 Co and 20 – 40 atm. Porous graphite electrode containing Ni and NiO serves as the inert electrodes.
Hydrogen and oxygen gases are bubbled through the anode and cathode, respectively.
Oxidation occurs at the anode: 2H + 4 OH (aq) 4 H O 2 2 4( ) ( ) e_g l_− −→ +
Reduction occurs at the cathode O (g) + 2 H O (l) + 4e 4 OH (aq)2 2 − −→
The overall reaction is 2H (g) + O (g) 2H O (l) 2 2 2→
The above reaction is the same as the hydrogen combustion reaction, however, they do not react directly ie., the oxidation and reduction reactions take place separately at the anode and cathode respectively like H - O2 2 fuel cell. Other fuel cells like propane –O2 and methane O2 have also been developed.
Corrosion
We are familiar with the rusting of iron. Have you ever noticed a green film formed on copper and brass vessels?. In both, the metal is oxidised by oxygen in presence of moisture. This redox process which causes the deterioration of metal is called corrosion. As the corrosion of iron causes damages to our buildings, bridges etc….it is important to know the chemistry of rusting and how to prevent it. Rusting of iron is an electrochemical process.
Electrochemical mechanism of corrosion
The formation of rust requires both oxygen and water. Since it is an electrochemical redox process, it requires an anode and cathode in different places on the surface of iron. The iron surface and a droplet of water on the surface as shown in figure (9.15) form a tiny galvanic cell. The region enclosed by water is exposed to low amount of oxygen and it acts as the anode. The remaining area has high amount of oxygen and it acts as cathode. So based on the oxygen content, an electro chemical cell is formed. corrosion occurs at the anode i,e,. in the region enclosed by the water as discussed below.
Fe(s) → Fe2+(aq)+2e– O2(g) + 4H+(aq)+4e– → 2H2O(I)
water
anodic site
cathodic site
Fe2+
O2
O2
Fe2O3• x H2O
e–
H2O Rust
iron
Figure 9.15 Rusting of iron
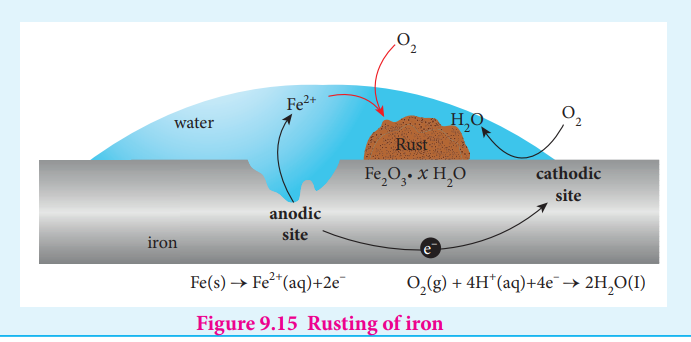
At anode (oxidation): Iron dissolves in the anode region
2Fe(s) 2Fe 2+
(aq)+4e - E V.→ = 0 44.
The electrons move through the iron metal from the anode to the cathode area where the oxygen dissolved in water, is reduced to water.
At Cathode (reduction) The reaction of atmospheric carbon dioxide with water gives carbonic acid which furnishes
the H + ions for reduction.
O2(g)+4H +
(aq)+4e -
2H2O ( ) → l E V = 1 23.
The electrical circuit is completed by the migration of ions through water droplet.
The overall redox reactions is, + 2+2Fe(s)+O (g)+4H (aq) 2Fe (aq) + 2H O( ) E 0.444 1.23 1.67V2 2 _l_→ = + =
The positive emf value indicates that the reaction is spontaneous.
Fe 2+ ions are further oxidised to Fe
3+ , which on further reaction with oxygen to form rust. 4Fe
2+ (aq)+O2(g)+4H
+ (aq) 4Fe
3+ (aq)+2H2O(l)→
3+ +2Fe (aq)+4H O(l) Fe O .H O(s) + 6H (aq)2 2 3 2→
Other metals such as aluminium, copper and silver also undergo corrosion, but at a slower rate than iron. For example, let us consider the reduction of aluminium,
Al(s) Al (aq)+3e→ + −3
Al 3+ , which reacts with oxygen in air to forms a protective coating of Al2O3 . This coating act as
a protective film for the inner surface. So,further corrosion is prevented.
Protection of metals form corrosion This can be achieved by the following methods. i. Coating metal surface by paint. ii. Galvanizing - by coating with another metal such as zinc. zinc is stronger reducing agent
than iron and hence it can be more easily corroded than iron. i.e., instead of iron, the zinc is oxidised.
iii. Cathodic protection - In this technique, unlike galvanising the entire surface of the metal to be protected need not be covered with a protecting metal. Instead, metals such as Mg or zinc which is corroded more easily than iron can be used as a sacrificial anode and the iron material acts as a cathode. So iron is protected, but Mg or Zn is corroded.
Passivation - The metal is treated with strong oxidising agents such as concentrated HNO3 . As a result, a protective oxide layer is formed on the surface of metal. Alloy formation - The oxidising tendency of iron can be reduced by forming its alloy with other more anodic metals.
Example, stainless steel - an alloy of Fe and Cr .
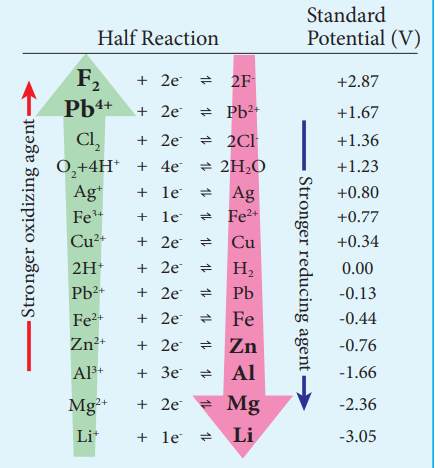
Electrochemical series
We have already learnt that the standard single electrode potentials are measured using standard hydrogen electrode. The standard electrode potential at 298K for various metal - metal ion electrodes are arranged in the decreasing order of their standard reduction potential values as shown in the figure.
This series is called electrochemical series.
The standard reduction potential (E ) is a measure of the oxidising
tendency of the species. The greater the E value, greater is the tendency shown by the species to accept electrons and undergo reduction. So higher the (E∞) Value, lesser is the tendency to undergo corrosion
EVALUATION
Choose the correct answer:
1. The number of electrons that have a total charge of 9650 coulombs is
a) 6 22 1023. × b) 6 022 1024. ×
c) 6 022 1022. × c) 6 022 10 34. × −
2. Consider the following half cell reactions:
Mn + 2e Mn E -1.18V2+ − → =
2 3+ -Mn Mn e E = -1.51V+ → +
The E for the reaction 2+ 3+3Mn Mn+2Mn ,→ and the possibility of the forward reaction are respectively. a) 2.69V and spontaneous b) -2.69 and non spontaneous c) 0.33V and Spontaneous d) 4.18V and non spontaneous
3. The button cell used in watches function as follows
Zn (s) + Ag O (s) + H O ( ) 2 Ag (s) + Zn (aq) + 2OH (2 2 l
2+ − aq) the half cell potentials are 2 2Ag O (s) + H O ( ) + 2e 2Ag (s) + 2 OH (aq) E 0.34V_l_ − −→ = The cell potential will be
Half Reaction Standard Potential (V)
-3.05
-2.36
-1.66 -0.76 -0.44 -0.13
+0.34 +0.77 +0.80 +1.23 +1.36 +1.67
+2.87
0.00
St ro
ng er
o xi
di zi
ng a
ge nt
Stronger reducing agent
Li
F2 Pb4+
Cl2
O2+4H+
Ag+
Fe3+
Cu2+
2H+
Pb2+
Fe2+
Zn2+
Al3+
Mg2+
Li+
Mg Al Zn Fe Pb
Cu Fe2+
Ag 2H2O 2Cl-
Pb2+
2F-
H2
+ + + + + + + + + + +
+ 1e-
1e-
1e-
2e-
2e-
2e-
2e-
2e-
2e-
2e-
2e-
2e-
3e-
4e-
XII U9 Electro Chemistry.indd 62 2/19/2020 5:15:19 PM
63
a) 0.84V b) 1.34V c) 1.10V d) 0.42V
4. The molar conductivity of a 0.5 mol dm-3 solution of AgNO3 with electrolytic conductivity of 5 76 10 3 1. × − − S cm at 298 K is
a) 2.88 S cm mol2 -1 b) 2 -111.52 S cm mol
c) 0.086 S cm mol2 -1 d) 28.8 S cm mol 2 -1
Electrolyte KCl KNO3 HCl NaOAC NaCl
(S cm mol
–Λ 2 1− )
149.9 145.0 426.2 91.0 126.5
Calculate HOACΛ using appropriate molar conductances of the electrolytes listed above at infinite dilution in water at 25 Co .
a) 517.2 b) 552.7 c) 390.7 d) 217.5
6. Faradays constant is defined as a) charge carried by 1 electron b) charge carried by one mole of electrons c) charge required to deposit one mole of substance d) charge carried by 6 22 1010. × electrons.
7. How many faradays of electricity are required for the following reaction to occur
MnO Mn4 - 2+→
a) 5F b) 3F c) 1F d) 7F
8. A current strength of 3.86 A was passed through molten Calcium oxide for 41minutes and 40 seconds. The mass of Calcium in grams deposited at the cathode is (atomic mass of Ca is 40g / mol and 1F = 96500C).
a) 4 b) 2 c) 8 d) 6
9. During electrolysis of molten sodium chloride, the time required to produce 0.1mole of chlorine gas using a current of 3A is
a) 55 minutes b) 107.2 minutes c) 220 minutes d) 330 minutes
10. The number of electrons delivered at the cathode during electrolysis by a current of 1A in 60 seconds is (charge of electron = 1 6 10 19. × − C )
a) 236.22 10× b) 6 022 1020. × c) 3 75 1020. × d) 7 48 1023. ×
11. Which of the following electrolytic solution has the least specific conductance
a) 2N b) 0.002N c) 0.02N d) 0.2N
12. While charging lead storage battery
a) PbSO4 on cathode is reduced to Pb b) PbSO4 on anode is oxidised to PbO2
| Electrolyte | KCl | KNO3 | HCl | NaOAC | NaCl |
|---|---|---|---|---|---|
| Λ(S cm mol )–21 − | 149.9 | 145.0 | 426.2 | 91.0 | 126.5 |
64
c) 4PbSO on anode is reduced to Pb d) PbSO4 on cathode is oxidised to Pb
13. Among the following cells I) Leclanche cell II) Nickel – Cadmium cell III) Lead storage battery IV) Mercury cell Primary cells are
a) I and IV b) I and III c) III and IV d) II and III
14. Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because
a) Zinc is lighter than iron
b) Zinc has lower melting point than iron
c) Zinc has lower negative electrode potential than iron
d) Zinc has higher negative electrode potential than iron
15. Assertion : pure iron when heated in dry air is converted with a layer of rust. Reason : Rust has the composition Fe O3 4
a) if both assertion and reason are true and reason is the correct explanation of assertion.
b) if both assertion and reason are true but reason is not the correct explanation of assertion.
c) assertion is true but reason is false
d) both assertion and reason are false.
16. In H -O2 2 fuel cell the reaction occurs at cathode is
a) O (g) + 2H O + 4e 4OH (aq)2 2 ( )l − −→
b) H (aq) + OH (aq) H O (+ − → 2 l)
c) 2H (g) + O (g) 2H O (g)2 2 2→
d) H + e H + −→ 1
2 2
17. The equivalent conductance of M 36solution of a weak monobasic acid is 6 mho cm2
equivalent –1 and at infinite dilution is 400 mho cm2 equivalent –1. The dissociation constant of this acid is
a) 1 25 10 6. × − b) 66.25 10−× c) 1 25 10 4. × − d) 56.25 10−×
18. A conductivity cell has been calibrated with a 0.01M, 1:1 electrolytic solution (specific conductance (κ = S cm1 25 10
3 1 . × − − ) in the cell and the measured resistance was 800 W at
25C . The cell constant is,
a) 10 1 1− − c m b) 101 1 c m− c) 1 1 c m− d) 125.7 10−×
19. Conductivity of a saturated solution of a sparingly soluble salt AB (1:1 electrolyte) at 298K is 1 85 10 5 1. × − − S m . Solubility product of the salt AB at 298K Λm AB
S m mol( ) = × − −14 10 3 2 1 .
a) 125.7 10−× b) 1 32 10 12. × − c) 7 5 10 12. × − d) 121.74 10−×
20. In the electrochemical cell: Zn ZnSO (0.01M) CuSO (1.0M) Cu4 4 , the emf of this Daniel cell is E1. When the concentration of is changed to 1.0M and that CuSO4 changed to 0.01M, the emf changes to E2. From the above, which one is the relationship between E1 and E2?
a) E1 < E2 b) E1 > E2 c) E E2 1 d) E1 = E2
21. Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram below:
BrO BrO HBrO Br Br4 - 1.82V
3 - 1.5V 1.595V
2 1.0652V - → → → →
Then the species undergoing disproportionation is
a) Br2 b) BrO4 − c) BrO3
- d) HBrO
22. For the cell reaction
2Fe (aq) + 2l (aq) 2Fe (aq) + l (aq)3+ − +→ 2 2
E 0.24V cell o = at 298K. The standard Gibbs energy (∆, G∞) of the cell reactions is :
a) -46.32 KJ mol−1 b) -23.16 KJ mol−1 c) 46.32 KJ mol−1 d) 123.16 KJ mol−
23. A certain current liberated 0.504gm of hydrogen in 2 hours. How many grams of copper can be liberated by the same current flowing for the same time through copper sulphate solution
a) 31.75 b) 15.8 c) 7.5 d) 63.5
24. A gas X at 1 atm is bubbled through a solution containing a mixture of 1MY- and 1MZ- at 25 Co . If the reduction potential of Z>Y>X, then
a) Y will oxidize X and not Z b) Y will oxidize Z and not X
d) Y will oxidize both X and Z d) Y will reduce both X and Z
25. Cell equation : A + 2B A +2B;- 2+→
A + 2e A E V and log K = 15.6 at 300K2 100 34+ −→ = + . for cell reactions find E for
B B+ + →−_e_ (AIIMS – 2018)
a) 0.80 b) 1.26 c) -0.54 d) -10.94
Short Answer Questions
1. Define anode and cathode
2. Why does conductivity of a solution decrease on dilution of the solution
3. State Kohlrausch Law. How is it useful to determine the molar conductivity of weak electrolyte at infinite dilution.
4. Describe the electrolysis of molten NaCl using inert electrodes
5. State Faraday’s Laws of electrolysis
6. Describe the construction of Daniel cell. Write the cell reaction.
7. Why is anode in galvanic cell considered to be negative and cathode positive electrode?
8. The conductivity of a 0.01M solution of a 1 :1 weak electrolyte at 298K is 1.5 10 S cm-4× −1.
i) molar conductivity of the solution
ii) degree of dissociation and the dissociation constant of the weak electrolyte
Given that λ λ
cation
anion
= 248.2 S cm mol S cm mol
2 1
2 151 8
−
−= .
9. Which of 0.1M HCl and 0.1 M KCl do you expect to have greater Λ 0
m and why?
10. Arrange the following solutions in the decreasing order of specific conductance.
i) 0.01M KCl ii) 0.005M KCl iii) 0.1M KCl
iv) 0.25 M KCl v) 0.5 M KCl
11. Why is AC current used instead of DC in measuring the electrolytic conductance?
12. 0.1M NaCl solution is placed in two different cells having cell constant 0.5 and 0.25cm-1 respectively. Which of the two will have greater value of specific conductance.
13. A current of 1.608A is passed through 250 mL of 0.5M solution of copper sulphate for 50 minutes. Calculate the strength of Cu2+ after electrolysis assuming volume to be constant and the current efficiency is 100%.
14. Can Fe3+ oxidises bromide to bromine under standard conditions?
Given: E Fe Fe3+ 2+
= 0 771.
E V. Br Br2
− = 1 09.
15. Is it possible to store copper sulphate in an iron vessel for a long time?
Given : E Cu Cu2+
= 0 34. V and E V Fe Fe2+
= −0 44. .
16. Two metals M1 and M2 have reduction potential values of - V and +yV_x_ respectively.
Which will liberate H2 and H2SO4. 17. Reduction potential of two metals M1 and M2 are E V and E V
M M M M11 2
1 2
2 2 3 0 2+ += − = . .
Predict which one is better for coating the surface of iron. Given : E V Fe Fe2+
= −0 44.
18. Calculate the standard emf of the cell: Cd Cd Cu Cu 2+2+ and determine the cell reaction.
The standard reduction potentials of Cu Cu and Cd Cd2+ 2+ are 0.34V and -0.40 volts respectively. Predict the feasibility of the cell reaction.
19. In fuel cell H2 and O2 react to produce electricity. In the process, H2 gas is oxidised at the anode and 2O at cathode. If 44.8 litre of H2 at 25 Co and 1atm pressure reacts in 10 minutes, what is average current produced? If the entire current is used for electro deposition of Cu from Cu2+ , how many grams of deposited?
20. The same amount of electricity was passed through two separate electrolytic cells containing solutions of nickel nitrate and chromium nitrate respectively. If 2.935g of Ni was deposited in the first cell. The amount of Cr deposited in the another cell? Give : molar mass of Nickel and chromium are 58.74 and 52gm-1 respectively.
21. A copper electrode is dipped in 0.1M copper sulphate solution at 25 Co . Calculate the electrode potential of copper. [Given: E
Cu Cu2+ = 0 34. V ].
22. For the cell Mg (s) Mg (aq) Ag (aq) Ag (s), 2+ + calculate the equilibrium constant at 25 Co and maximum work that can be obtained during operation of cell. Given : E V and E V
Mg Mg Ag Ag2+ +
= − =2 37 0 80. . .
23. 8 2 1012. × litres of water is available in a lake. A power reactor using the electrolysis of water in the lake produces electricity at the rate of 2 106 1× − Cs at an appropriate voltage. How many years would it like to completely electrolyse the water in the lake. Assume that there is no loss of water except due to electrolysis.
24. Derive an expression for Nernst equation
25. Write a note on sacrificial protection.
26. Explain the function of H - O2 2 fuel cell.
27. Ionic conductance at infinite dilution of Al3+ and SO4 2- are 189 and 160 mho cm2 equiv-1.
Calculate the equivalent and molar conductance of the electrolyte Al (SO )2 4 3 at infinite dilution.
Simulating an Voltaic Cell
Step – 1
Open the Browser and type the URL given (or) Scan the QR Code. You will see the webpage as shown in
the figure.
Step – 2
Choose the metal electrode and appropriate electrolytic solution for both cathode and anode following the
on screen instructions. Now switch on the volt meter by clicking the red power switch. Now you can see
the flow of electrons and the emf value on the screen.
Step – 3
The above steps can be repeated by varying the concentrations of electrolytic solutions of cathode and
anode by selecting appropriate concentration from the list.
By using this tool you can construct an electrochemical
cell with using Ag/Cu/Zn electrodes and measure the emf of the cell. You can also learn how the concentra� on aff ects the emf value of the
cell.
Please go to the URL h� ps:// pages.uoregon.edu/tgreenbo/ voltaicCellEMF.html (or) Scan the QR codeon the right side
ICT Corner