Swiss chemist Alfred Werner was the first one to propose a theory of coordination compounds to explain the observed behaviour of them.
Let us consider the different coloured complexes of cobalt(III) chloride with ammonia which exhibit different properties as shown below.
| Complex | Color | No. of moles of AgCl precipitated on reaction of one mole of complex with excess Ag+ |
|---|---|---|
| CoCl3.6NH3 | Yellow | 3 |
| CoCl3.5NH3 | purple | 2 |
| trans - CoCl3.4NH3 | green | 1 |
| cis - CoCl3.4NH3 | violet | 1 |
In this case, the valences of the elements present in both the reacting molecules, cobalt(III) chloride and ammonia are completely satisfi ed. Yet these substances react to form the above mentioned complexes.
To explain this behaviour Werner postulated his theory as follows
-
Most of the elements exhibit, two types of valence namely primary valence and secondary valence and each element tend to satisfy both the valences.In modern terminology, the primary valence is referred as the oxidation state of the metal atom and the secondary valence as the coordination number. For example, according to Werner, the primary and secondary valences of cobalt are 3 and 6 respectively.
-
Th e primary valence of a metal ion is positive in most of the cases and zero in certain cases. Th ey are always satisfi ed by negative ions. For example in the complex CoCl3.6NH3, Th e primary valence of Co is +3 and is satisfi ed by 3Cl- ions.
-
Th e secondary valence is satisfi ed by negative ions, neutral molecules, positive ions or the combination of these. For example, in CoCl3.6NH3 the secondary valence of cobalt is 6 and is satisfi ed by six neutral ammonia molecules, whereas in CoCl3.5NH3 the secondary valence of cobalt is satisfi ed by fi ve neutral ammonia molecules and a Cl- ion.
-
According to Werner, there are two spheres of attraction around a metal atom/ion in a complex. Th e inner sphere is known as coordination sphere and the groups present in this sphere are fi rmly attached to the metal. Th e outer sphere is called ionisation sphere. Th e groups present in this sphere are loosely bound to the central metal ion and hence can be separated into ions upon dissolving the complex in a suitable solvent.
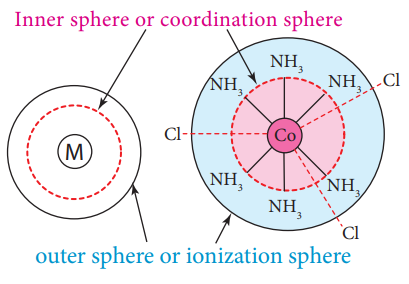
inner and outer spheres of attraction in coordination compounds
Figure 5.1 inner and outer spheres of attraction in coordination compounds
- The primary valences are non-directional while the secondary valences are directional. The geometry of the complex is determined by the spacial arrangement of the groups which satisfy the secondary valence. For example, if a metal ion has a secondary valence of six, it has an octahedral geometry. If the secondary valence is 4, it has either tetrahedral or square planar geometry.
The following table illustrates the Werner’s postulates.
| Complex | Groups satisfy the secondary valence(non-ionaisable, inner coordination sphere) | No.of ionisable Clions in the complex(outer coordination sphere) | No. of moles of AgCl formed = no. of moles of ionisable Cl |
|---|---|---|---|
| CoCl3.6NH3 | 6NH3 | 3 cl- | 3 Agcl |
| CoCl3.5NH3 | 5 NH3&1 Cl- | 2Cl- | 2 AgCl |
| CoCl3.4NH3 | 4 NH3&2 Cl- | 1Cl- | 1 AgCl |
| CoCl3.4NH3 | 4 NH3&2 Cl- | 1Cl- | 1 AgCl |
Limitations of Werner’s theory:
Even though, Werner’s theory was able to explain a number of properties of coordination compounds, it does not explain their colour and the magnetic properties.
| Evaluate yourself 1:When a coordination compound CrCl3.4H2O is mixed with silver nitrate solution, one moleof silver chloride is precipitated per mole of the compound. There are no free solvent molecules in that compound. Assign the secondary valence to the metal and write the structural formula of the compound. |
|---|