The integrated rate equation:
We have just learnt that the rate of change of concentration of the reactant is directly proportional to that of concentration of the reactant. For a general reaction,
A →products
The rate law is
Rate = -d[A]/dt=k [A]x Where k is the rate constant, and x is the order of the reaction. The above equation is a differential equation, -d[A]/dt , so it gives the
rate at any instant. However, using the above expression, we cannot answer questions such as how long will it take for a specific concentration of A to be used up in the reaction? What will be the concentration of
reactant after a time ‘ t ’?. To answer such questions, we need the integrated form of the above rate law which contains time as a variable.
Integrated rate law for a first order reaction
A reaction whose rate depends on the reactant concentration raised to the first power is called a first order reaction. Let us consider the following first order reaction,
A →prosuct Rate law can be expressed as
Rate = k [A]1
Where, k is the first order rate constant. -d[A]/dt=k[A]1 ⇒ = -d[A]/[A]= k dt …(1)
Integrate the above equation between the limits of time t = 0 and time equal to t, while the concentration varies from the initial concentration [A0 ] to [A] at the later time.
∫[A] [A0] -d[A0]/[A]=k∫tdt
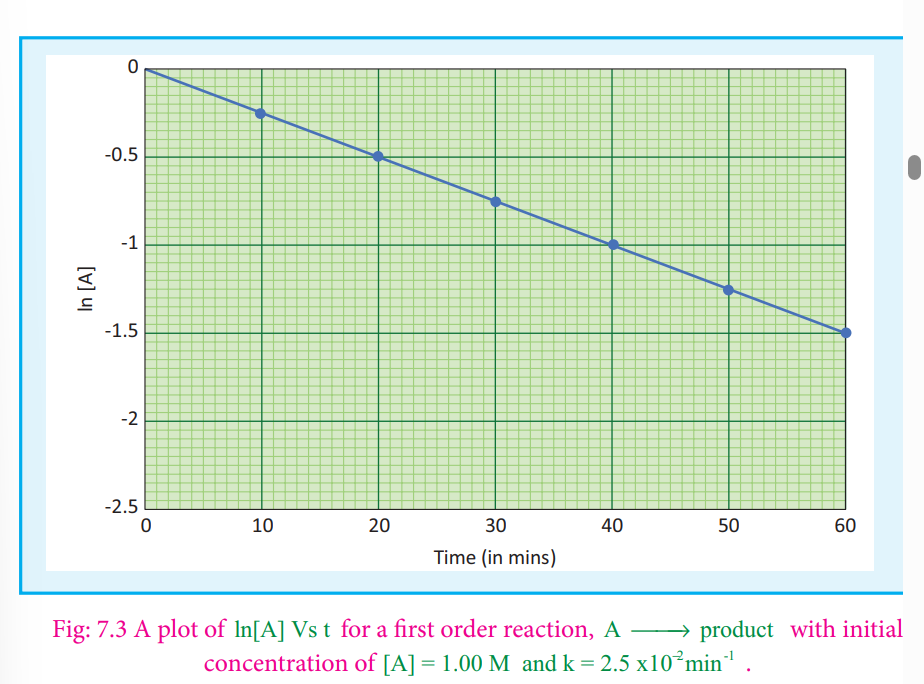
ln([A0]/[A]) = kt …(2)
This equation is in natural logarithm. To convert it into usual logarithm with base 10, we have to multiply the term by 2.303.
2.303 log [A0]/[A] = kt
Equation (2) can be written in the form y = mx + c as below
⇒y = c + mx
If we follow the reaction by measuring the concentration of the reactants at regular time interval‘t’, a plot of ln[A] against ‘t’ yields a straight line with a negative slope.From this, the rate constant is calculated.
Examples for the first order reaction
(i) Decomposition of dinitrogen pentoxide
N2O5(g)—> 2NO 2(g) + 1/2 O2(g).
(ii) Decomposition of sulphurylchloride; SO2Cl2(l)—> SO2(g)+ Cl2(g).
(iii) Decomposition of the H2O2 in aqueous solution; H2O2(aq)—> H2O(l)+1/2 O2(g).
(iv) Isomerisation of cyclopropane to propene.
Pseudo first order reaction: Kinetic study of a higher order reaction is difficult to follow, for example, in a study of a second
order reaction involving two different reactants; the simultaneous measurement of change in the concentration of both the reactants is very difficult. To overcome such difficulties, A second order reaction can be altered to a first order reaction by taking one of the reactant in large excess, such reaction is called pseudo first order reaction. Let us consider the acid hydrolysis of an ester,
$\ce{CH3COOHCH3(aq)+H2O(l)->[{H+}] CH3COOH(aq)+CH3OH}$
Rate = k [CH3COOCH3] [H2O]
If the reaction is carried out with the large excess of water, there is no significant change in the concentration of water during hydrolysis. i.e.,concentration of water remains almost a constant.
Now, we can define k[H2 O] = k’; Therefore the above rate equation becomes
Rate = k’[CH3COOCH3]
Thus it follows first order kinetics.
Integrated rate law for a zero order reaction:
A reaction in which the rate is independent of the concentration of the reactant over a
wide range of concentrations is called as zero order reactions. Such reactions are rare. Let us consider the following hypothetical zero order reaction.
A →product
The rate law can be written as,
Rate = k [A]0
−d[A]/ dt
=k (1)
∴ =([A]0=1)
⇒ − d[A] = k dt
Integrate the above equation between the limits of [A ] 0 at zero time and [A] at some later
time ’t',
− ∫d[A] = k ∫dt
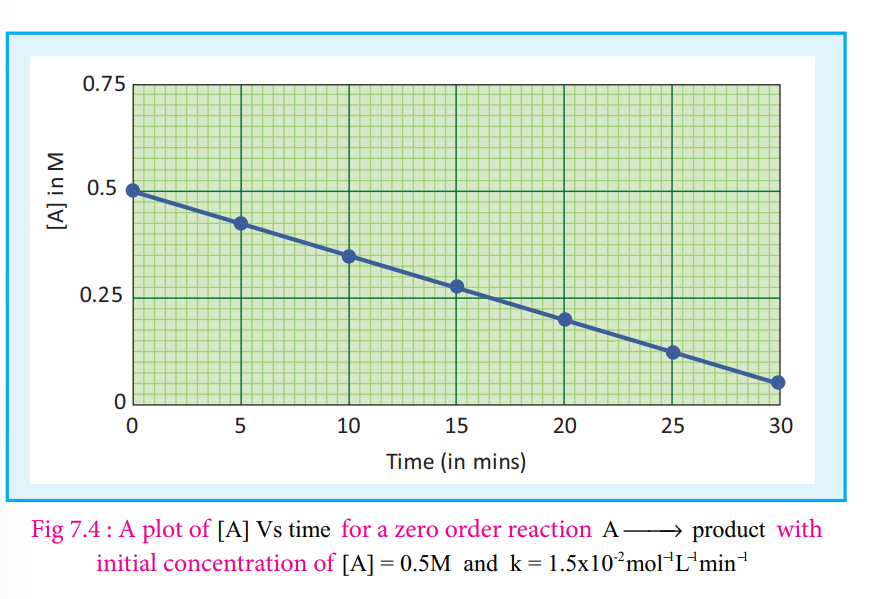
k=[A0]-[A]/t
Equation (2) is in the form of a straight line y = mx + c
Ie., [A]=[A]/t ⇒ y = c + mx
A plot of [A] Vs time gives a straight line with a slope of −k and y - intercept of [A0 ].
Examples for a zero order reaction: 1. Photochemical reaction between H2 and I2
$\ce{H2(g)+Cl2(g) ->[{hv}] 2HCL(g)}$
2. Decomposition of N2O on hot platinum surface
N2O(g) ⇀↽N2(g)+1/2O2(g)
3. Iodination of acetone in acid medium is
zero order with respect to iodine.
$\ce{CH3COCH3+l2(g) ->[{h+}] ICH2COCH3+HI}$
Rate = k [CH3COCH3] [H+]
General rate equation for a nth order reaction involving one reactant [A].
A →product
Rate law − =d[A]/dt=k[A]n
Consider the case in which n≠1,
integration of above equation between [A0] and [A] at time t = 0 and t = t
respectively gives 1/[A]n-1 - 1/[A]n-1 =(n-1)kt