Transport of gases
Transport of oxygen
Molecular oxygen is carried in blood in two ways bound to haemoglobin within the red blood cells and dissolved in plasma. Oxygen is poorly soluble in water, so only 3% of the oxygen is transported in the dissolved form. 97% of oxygen binds with haemoglobin in a reversible manner to form oxyhaemoglobin (HbO2). The rate at which haemoglobin binds with O2 is regulated by the partial pressure of O2. Each haemoglobin carries maximum of four molecules of
oxygen. In the alveoli high pO2, low pCO2, low temperature and less H+ concentration, favours the formation of oxyhaemoglobin, whereas in the tissues low pO2, high pCO2, high H+ and high temperature favours the dissociation of oxygen from oxyhaemoglobin.
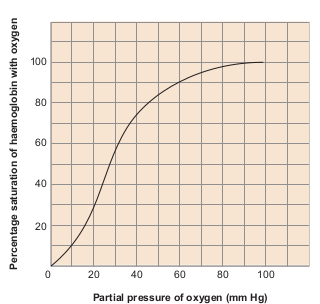
A sigmoid curve (S–shaped) is obtained when percentage saturation of haemoglobin with oxygen is plotted
against pO2. This curve is called oxygen haemoglobin dissociation curve (Figure 6.7). This S–shaped curve has a steep slope for pO2 values between 10 and 50mmHg and then flattens between 70 and 100 mm Hg.
Under normal physiological conditions, every 100mL of oxygenated blood can deliver about 5mL of O2 to the tissues.
Transport of Carbondioxide
Blood transports CO2 from the tissue cells to the lungs in three ways
i. Dissolved in plasma About 7 – 10% of CO2 is transported in a dissolved form in the plasma.
ii. Bound to haemoglobin About 20 – 25% of dissolved CO2 is bound and carried in the RBCs as carbaminohaemoglobin (Hb CO2) CO2 + Hb == Hb CO2
iii. As bicarbonate ions in plasma about 70% of CO2 is transported as bicarbonate ions
This is influenced by pCO2 and the degree of haemoglobin oxygenation. RBCs contain a high concentration of the enzyme, carbonic anhydrase, whereas small amounts of carbonic anhydrase is present in the plasma.
At the tissues the pCO2 is high due to catabolism and diffuses into the blood to form HCO3
– and H+ ions. When CO2
diffuses into the RBCs, it combines with water forming carbonic acid (H2CO3) catalyzed by carbonic anhydrase. Carbonic acid is unstable and dissociates into hydrogen and bicarbonate ions.
Carbonic anhydrase facilitates the reaction in both directions.

The HCO3 – moves quickly from the RBCs into the plasma, where it is carried to the lungs. At the alveolar site where pCO2 is low, the reaction is reversed leading to the formation of CO2 and water. Thus CO2 trapped as HCO3 – at the tissue level is transported to the alveoli and released out as CO2. Every 100mL of deoxygenated blood delivers 4mL of CO2 to the alveoli for elimination.
Bohr effect and Haldane effect
Increase in pCO2 and decrease in pH decrease the affinity of haemoglobin for oxygen and shifts the oxyhaemoglobin dissociation curve to the right and facilitates unloading of oxygen from hemoglobin in the tissue. This effect of pCO2 and pH on the oxyhaemoglobin dissociation curve is called the Bohr effect.
The Haldane effect, on the other hand describes how oxygen concentrations determines hemoglobin’s affinity for carbon dioxide. The amount of carbon dioxide transported in blood is remarkably affected by the degree of oxygenation of the blood. The lower the partial pressure of O2 lower is the affinity of haemoglobin saturation with oxygen hence more CO2 is carried in the blood. This phenomenon is called Haldane effect. This effects CO2 exchanges in both the tissues and lungs.
In the lungs the process is reversed as the blood moves through the pulmonary capillaries, its pCO2 declines from 45mm Hg to 40mm Hg. For this to occur carbondioxide is freed from HCO3- ions and Cl- ions moves in to the plasma and reenters the RBC and binds with H+ to form carbonic acid which dissociates into CO2 and water. This CO2 diffuses along its partial gradient from the blood to the alveoli (Figure 6.8).