
Temperature and heat play very important role in everyday life. All species can function properly only if its body is maintained at a particular temperature. In fact life on Earth is possible because the Sun maintains its temperature. Understanding the meaning of temperature and heat are very crucial to understand the nature. Thermodynamics is a branch of physics which explains the phenomena of temperature, heat etc. The concepts presented in this chapter will help us to understand the terms ‘hot’ and ‘cold’ and also differentiate heat from temperature. In thermodynamics, heat and temperature are two different but closely related parameters.
Meaning of heat
When an object at higher temperature is placed in contact with another object at lower temperature, there will be a spontaneous flow of energy from the object at higher temperature to the one at lower temperature. This energy is called heat. This process of energy transfer from higher temperature object to lower temperature object is called heating. Due to flow of heat sometimes the temperature of the body will increase or sometimes it may not increase.
There is a misconception that heat is a quantity of energy. People often talk ‘this
water has more heat or less heat’. These words are meaningless. Heat is not a quantity. Heat is an energy in transit which flows from higher temperature object to lower temperature object. Once the heating process is stopped we cannot use the word heat. When we use the word ‘heat’, it is the energy in transit but not energy stored in the body.
Note There is a misconception that heat is a quanity of energy. People oftens talk ’this water has more heat or less heat’. These words are meaningless. Heat is not a quanity. Heat is an energy in transit which flows from higher temperature object to lower temperature object.Once the heating process is stopped we cannot use the word heat. when we use the word ‘heat’, it is the energy in transit but not energy stored in the body.
EXAMPLE 8.1 a. ‘A lake has more rain’. b. ‘A hot cup of coffee has more heat’.
What is wrong in these two statements?
Solution a. When it rains, lake receives water
from the cloud. Once the rain stops, the lake will have more water than before raining. Here ‘raining’ is a process which brings water from the cloud. Rain is not a quantity rather it is water in transit. So the statement ‘lake has more rain’ is wrong, instead the ‘lake has more water’ will be appropriate.
b. When heated, a cup of coffee receives heat from the stove. Once the coffee is taken from the stove, the cup of coffee has more internal energy than before. ‘Heat’ is the energy in transit and which flows from an object at higher temperature to an object at lower temperature. Heat is not a quantity. So the statement ‘A hot cup of coffee has more heat’ is wrong, instead ‘coffee is hot’ will be appropriate.
Meaning of work
When you rub your hands against each other the temperature of the hands increases. You have done some work on your hands by rubbing. The temperature of the hands increases due to this work. Now if you place your hands on the chin, the temperature of the chin increases. This is because the hands are at higher temperature than the chin. In the above example, the temperature of hands is increased due to work and temperature of the chin is increased due to heat transfer from the hands to the chin. It is shown in the Figure 8.1
By doing work on the system, the temperature in the system will increase and sometimes may not. Like heat, work is also
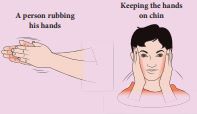
Figure 8.1 Difference between work and heat
not a quantity and through the work energy is transferred to the system . So we cannot use the word ‘the object contains more work’ or ‘less work’.
Either the system can transfer energy to the surrounding by doing work on surrounding or the surrounding may transfer energy to the system by doing work on the system. For the transfer of energy from one body to another body through the process of work, they need not be at different temperatures.
Meaning of temperature
Temperature is the degree of hotness or coolness of a body. Hotter the body higher is its temperature. The temperature will determine the direction of heat flow when two bodies are in thermal contact.
The SI unit of temperature is kelvin (K).
In our day to day applications, Celsius (˚C) and Fahrenheit (°F) scales are used.
Temperature is measured with a thermometer. The conversion of temperature from one scale to other scale is given in Table 8.1
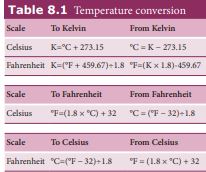
Table 8.1 Temperature conversion Scale To Kelvin From Kelvin