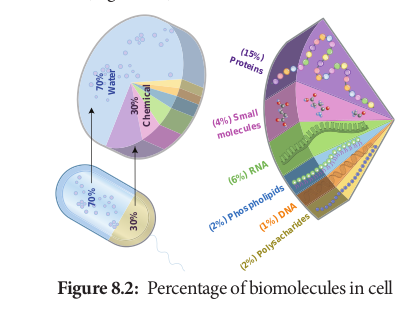
Water is the most abundant component in living organisms. Life on earth is inevitably linked to water. Water makes up 70% of human cell and upto 95% of mass of a plant cell (Figure 8.2).
Chemistry of Water
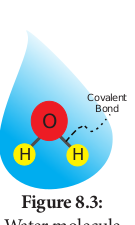
Water is a tiny polar molecule that can readily pass through membranes. Two electronegative atoms of oxygen share a hydrogen bonds of two water molecule. Thus, they can stick together by cohesion and results in lattice formation (Figure 8.3).
Properties of Water
- Adhesion and cohesion propert.
- High latent heat of vaporisatio.
- High melting and boiling poin.
- Universal solven.
- Specific heat capacity